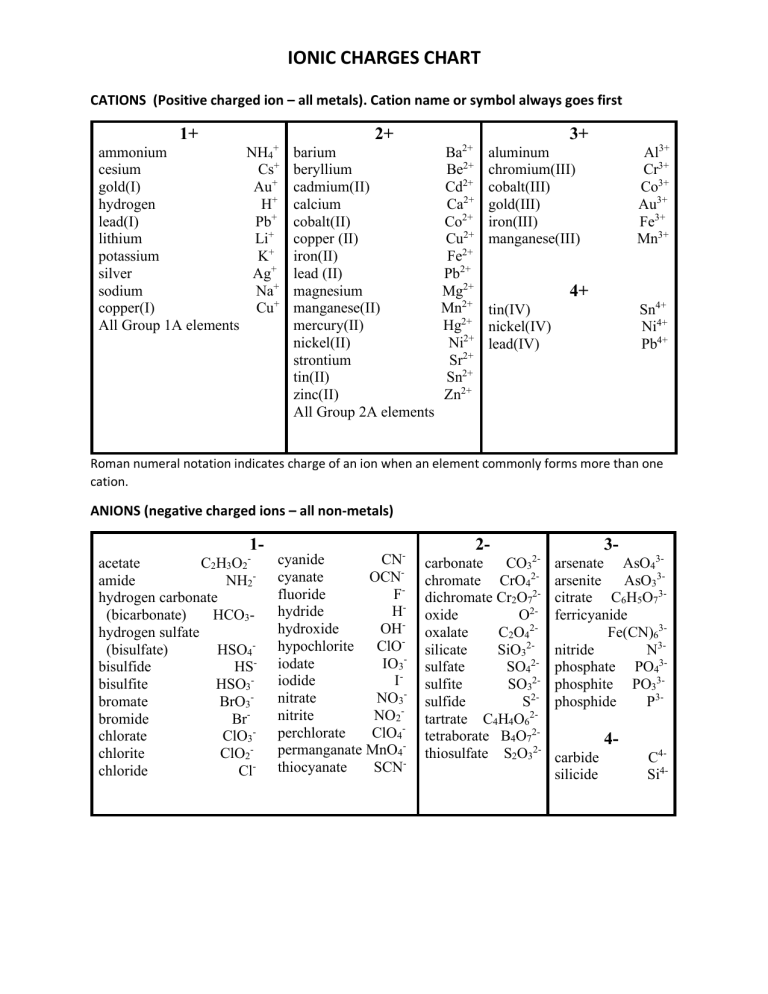
IONIC CHARGES CHART CATIONS (Positive charged ion – all metals). Cation name or symbol always goes first 1+ 2+ + ammonium NH4 cesium Cs+ gold(I) Au+ hydrogen H+ lead(I) Pb+ lithium Li+ potassium K+ silver Ag+ sodium Na+ copper(I) Cu+ All Group 1A elements 3+ barium beryllium cadmium(II) calcium cobalt(II) copper (II) iron(II) lead (II) magnesium manganese(II) mercury(II) nickel(II) strontium tin(II) zinc(II) All Group 2A elements 2+ Ba Be2+ Cd2+ Ca2+ Co2+ Cu2+ Fe2+ Pb2+ Mg2+ Mn2+ Hg2+ Ni2+ Sr2+ Sn2+ Zn2+ Al3+ Cr3+ Co3+ Au3+ Fe3+ Mn3+ aluminum chromium(III) cobalt(III) gold(III) iron(III) manganese(III) 4+ Sn4+ Ni4+ Pb4+ tin(IV) nickel(IV) lead(IV) Roman numeral notation indicates charge of an ion when an element commonly forms more than one cation. ANIONS (negative charged ions – all non-metals) 1- acetate C2H3O2 amide NH2hydrogen carbonate (bicarbonate) HCO3hydrogen sulfate (bisulfate) HSO4bisulfide HSbisulfite HSO3bromate BrO3bromide Brchlorate ClO3chlorite ClO2chloride Cl- cyanide CNcyanate OCNfluoride Fhydride Hhydroxide OHhypochlorite ClOiodate IO3iodide Initrate NO3nitrite NO2perchlorate ClO4permanganate MnO4thiocyanate SCN- 2- 32- carbonate CO3 chromate CrO42dichromate Cr2O72oxide O2oxalate C2O42silicate SiO32sulfate SO42sulfite SO32sulfide S2tartrate C4H4O62tetraborate B4O72thiosulfate S2O32- arsenate AsO43arsenite AsO33citrate C6H5O73ferricyanide Fe(CN)63nitride N3phosphate PO43phosphite PO33phosphide P3- 4carbide silicide C4Si4-