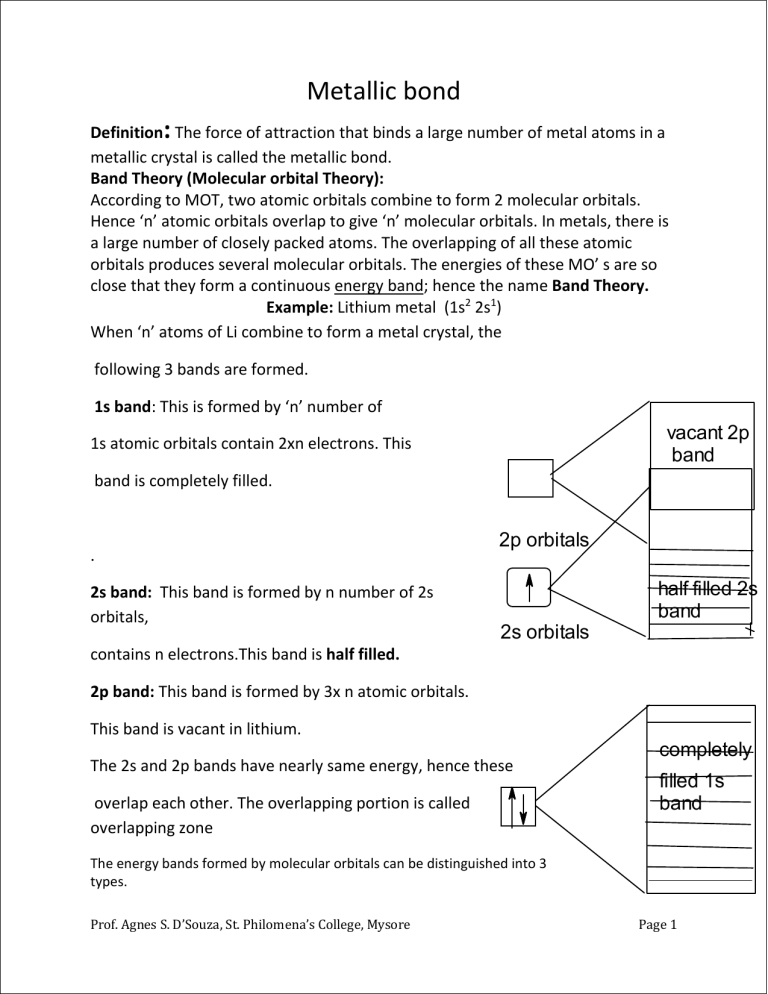
Metallic bond Definition: The force of attraction that binds a large number of metal atoms in a metallic crystal is called the metallic bond. Band Theory (Molecular orbital Theory): According to MOT, two atomic orbitals combine to form 2 molecular orbitals. Hence ‘n’ atomic orbitals overlap to give ‘n’ molecular orbitals. In metals, there is a large number of closely packed atoms. The overlapping of all these atomic orbitals produces several molecular orbitals. The energies of these MO’ s are so close that they form a continuous energy band; hence the name Band Theory. Example: Lithium metal (1s2 2s1) When ‘n’ atoms of Li combine to form a metal crystal, the following 3 bands are formed. 1s band: This is formed by ‘n’ number of vacant 2p band 1s atomic orbitals contain 2xn electrons. This band is completely filled. . 2s band: This band is formed by n number of 2s orbitals, 2p orbitals half filled 2s band 2s orbitals contains n electrons.This band is half filled. 2p band: This band is formed by 3x n atomic orbitals. This band is vacant in lithium. The 2s and 2p bands have nearly same energy, hence these overlap each other. The overlapping portion is called overlapping zone completely filled 1s band The energy bands formed by molecular orbitals can be distinguished into 3 types. Prof. Agnes S. D’Souza, St. Philomena’s College, Mysore Page 1 1. Conduction band 2.valence band 3.forbidden band or energy gap 1.Conduction band: The lowest unoccupied or partially filled energy band is called conduction band. 2.Valence band: The highest occupied energy band (completely filled) is called valence band. It is the non-conduction band. 3.Forbidden zone or forbidden gap: (Eg): The conduction band and valence band are separated by an energy gap, which prevents the promotion of electrons from lower to a higher band. This gap is called forbidden gap or forbidden zone.(Eg) Electrical properties of metals insulators and semiconductor. The electrical properties of metals, insulators and semiconductors depend on the number of electrons present in the conduction band and the width of the energy gap. Vacant Conduction band Partially filled conduction band c Conduction band large energy gap Conduction band co c small Eg Valence band Valance band Valence band Valence band Metals. Insulators semiconductors Energy Bands Metals: In metals (conductors) valence band is completely filled and the conduction band is partially filled. Small energy is sufficient to promote the electrons to the vacant levels within the same band. This movement of electrons in the conduction band gives rise to conduction. (In alkaline earth metals the valence band and conduction band actually overlap resulting in a partially filled upper –band) The electrical conductivity of a metal decreases with increase in temperature. This is because on increasing the temperature, thermal energy of atoms increases. Hence the vibrational motion of atoms (kernels) increases, which hinders the free movement of electrons. Prof. Agnes S. D’Souza, St. Philomena’s College, Mysore Page 2 Insulators: Insulators have very low conductance or zero conductance at room temperature. E.g. rubber, diamonds, wool etc. In the insulators, the valence band is completely filled and conduction band is vacant. The V.B and C.B are separated by large energy gap (Eg >6ev). No electron from valence band can cross over to conduction band at room temperature, even if the electric field is applied. High energy is required to promote the electron from V.B to C.B. crossing the large energy gap. This energy is not available at room temperature. Hence they are non-conductors at room temperature. Semiconductors: Semiconductors are materials, whose conductance lie in between that of good conductors and insulators. Eg. Silicon, germanium, Gallium arsenide etc. Semiconductors have filled valence band and empty conduction band, separated by a narrow energy gap. The Eg values lie in the range of 2-3eV. Hence electrons can be promoted from the V.B to C.B by the absorption of thermal energy at room temperature. As the temperature increases more and more electrons are excited from V.B to C.B. Hence the conductance increases with increase in temperature. Semiconductors are of two types. 1. Intrinsic semiconductors 2. Extrinsic semiconductors 1. Intrinsic semiconductors: Semiconductors in their extremely pure form are called intrinsic semiconductors. Eg. Si, Ge 2. Extrinsic semiconductors: Extrinsic semi conductors are the materials whose semiconducting properties are due to the addition of an extremely small amount of impurity atoms. The impurity atoms are called dopants. The impurity atoms present is usually one atom per 109 atoms of the host. These are of two types. (a) n-type extrinsic semiconductors: These are obtained by adding ( doping ) a small number of pentavalent atoms like P, AS, Sb etc. to pure elements like Si or Ge. Each Si atom forms 4 covalent bonds with 4 neighbouring Si atoms using its four valence electrons. Its 5th electron is left unused. Thus there is an extra electron at each Si atom. These loosely bound extra electrons can be excited easily from the balance band to the conduction band when an electric field or thermal energy is applied. Such semi conductors ‘doped’ with pentavalent impurity atom are called n-type semiconductors because negatively charged electrons are responsible for conduction. Prof. Agnes S. D’Souza, St. Philomena’s College, Mysore Page 3 vacant 2p band 2p orbitals half filled 2s band 2s orbitals completely filled 1s band Prof. Agnes S. D’Souza, St. Philomena’s College, Mysore Page 4 The excess loosely bound electrons occupy delocalized level called donor level just below the conduction band of Ge crystal. Electrons can be easily excited from donor band to conduction band. As the number of electrons in the conduction band increases, the conductance also increases. Note: Fermi level is defined as an energy level, below which all the energy states are filled Empty C. band — e _ e _ e _ e Empty C. Band conduction band _ e- e ------------------------------------------------- Donor impurity level --------------------------------------- fermilevel Eg Eg ENERGY Filled V. band Energy level: n-type semiconductor O OO O O O O Filled V.Band bbbbvvvvvalenm p-type semiconductor V>bv.vaadsvavvvb (b) p-type extrinsic semiconductors: These are obtained by adding trivalent bbbbababand atoms like B, Al, Ga, In to the parent Si or Ge. Each boron atom added forms 3 covalent bonds with 3 silicon atoms, while it is bonded to the fourth Si atom by one – electron bond. This electron deficiency creates a positive hole or vacancy at the site where the electron is missing. These holes occupy an energy level called acceptor level close to the filled valence band of silicon. The hole formed may be filled up by an electron from neighbouring atoms, thereby creating a positive hole there. In this way, the hole moves through the crystal. The movement of holes is thus regarded as the movement of positive electric charge. The holes move in a direction opposite to the motion of electrons. Since the carriers are positively charged holes, this type of semiconductors is called p-type semiconductors. Prof. Agnes S. D’Souza, St. Philomena’s College, Mysore Page 5 Superconductors: Super conductors are the materials which offer no resistance to the flow of electric current through them. Super conductivity was discovered by Kammerlingh Onnes in 1913. Most of the metals become superconducting at 4K. The critical temperature of the superconductors is the maximum temperature below which a material exhibits superconductivity. Eg. YBa2Cu3O9 (93K) Tl2Ba2Ca2Cu3O10 (125K) Uses 1. used in microwave communications as filters and antennas 2. In the levitation transportation 3. In medicine, to detect weak magnetic signals from heart and brain. Hydrogen Bonding Definition: The electrostatic force of attraction between H-atom and an electronegative atom present in a molecule of the same substance, or present in a molecule of different substance or present within the same molecule, is known as a hydrogen bond. 𝛿+ 𝛿_ 𝛿+ 𝛿__ 𝛿+ 𝛿__ 1. H – F…….H - F……..H –F Hydrogen bonding is represented by dotted line. The strength of hydrogen bond is about 1040kJ /mol while that of covalent bond is 400kJ/mol. Therefore hydrogen bond is much weaker than covalent bond. The condition for the formation of a hydrogen bond: 1. Presence of highly electronegative atom: The molecule having hydrogen bonding should have a highly electronegative atom like N, O or F directly linked to the hydrogen atom. 2. Presence of atom with small size: The highly electronegative atom should be of small size. Prof. Agnes S. D’Souza, St. Philomena’s College, Mysore Page 6 Types of hydrogen bonding: 1. Inter-molecular hydrogen bonding: The electro static attraction between the hydrogen atom of a molecule and the electronegative atom of another molecule of the same substance is called inter-molecular hydrogen bonding. Water, hydrogen fluoride and ammonia show this type of bonding. Example: 𝛿+ 𝛿_ 𝛿 + 𝛿__ 1. H – F…….H - F……..H –F 𝛿+ 𝛿__ 2) Intramolecular hydrogen bonding: The electrostatic force of attraction between the hydrogen atom and electronegative atom of the same molecule is called intramolecular hydrogen bonding. Example: o-nitrophenol, o-chlorophenol, salicylaldehyde.H Note: p-nitro phenol and p-chloro phenol do not show any intra molecular hydrogen bonding due to the distance between the 2 groups. On the other hand, they show usual inter molecular hydrogen bonding as shown below. Give reason: Ortho derivatives are more volatile than the para derivatives. Or the boiling point of para derivative is higher than ortho derivatives. Answer: As a result of intermolecular hydrogen bonding para molecules undergo association which results in an increase in the boiling point. In ortho, on account of intra molecular hydrogen bonding, no such association is possible. Consequences of hydrogen bonding : 1. High melting and boiling point: The compounds having hydrogen bonding show abnormally high melting and boiling points. 2.The physical state of H2O and H2S: Hydrogen bonding is responsible for the association of molecules in water. hence it exists as a liquid. Since sulphur atom is less electronegative than oxygen atom t her is no hydrogen bonding in hydrogen sulphide; it exists as a gas. Prof. Agnes S. D’Souza, St. Philomena’s College, Mysore Page 7 2. Association: The molecules of carboxylic acids exist as dimers because of the hydrogen bonding. The molecular masses of such compounds are found to be double than those calculated from their simple formulae. For example, the molecular mass of acetic acid is found to be 120. 3 Solubility: The solubility of organic compounds in water is due to the formation of H-bonds between H2O molecules and organic compounds. For example, lower alcohols are soluble in water because of the hydrogen bonding between water and alcohol molecules as shown below, 4. Crystal structure: As a result of hydrogen bonding, chain structure, sheet structure and threedimensional net work are formed in the crystals structures. 5. Water shows some unique properties because of hydrogen bonding. Anomalous properties of water: a. High boiling point of water. Water has a high boiling point.This is because a large number of water molecules are associated through hydrogen bonding. This gives water (H2O)n the polymerized clusters. In order to break these H-bonds, high energy is required. So the boiling point of water is high. b. Water is a gas where as hydrogen sulphide is a gas: Though both the water and H2S are the hydrides of the elements of the same group, water is liquid. This is because sulphur atom does not possess the desired electronegativity to indulge in hydrogen bonding. H2S remain unassociated c. The solubility of certain covalent compounds in water: H-bonding accounts for the solubility of certain covalent compounds in water.E.g., lower alcohols are soluble in water because these molecules form H- bonding in water. d.The density of ice is less than water: As ice is solid, the molecules are rigidly held in space. Each oxygen atom is tetrahedrally surrounded by four hydrogen atoms, two by covalent bonds and other 2 by H-bonds. The H-bonds are longer (weaker) bonds than covalent bonds. This arrangement gives rise to open-cage-structure with a lot of holes or open space between the atoms. These vacant spaces are responsible for its lesser density. e. Water has a maximum density at 40C: As water is heated from 00C, hydrogen bonds continue to break and the molecules come closer and closer. This leads to contraction and thereby increases its density. Up to 40C, this contraction will be more than the expansion due to heating effect. Prof. Agnes S. D’Souza, St. Philomena’s College, Mysore Page 8 Prof. Agnes S. D’Souza, St. Philomena’s College, Mysore Page 9

