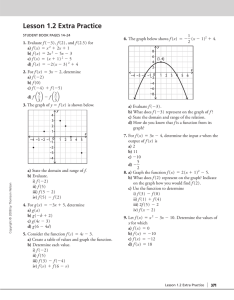
History of atom 01 02 03 04 Democritus John Dalton J.J Thomson Robert A. Millikan Eugen Goldstein DIRECTIONS 05 06 07 James Chadwick Ernest Rutherford 01 / 7 Democritus John Dalton J.J. Thomson Robert A. Millikan Eugen Goldstein James Chadwick Ernest Rutherford Democritus He was actually not a scientist but he was a Greek philosopher and he wanted to know the natural world. Greek philosophers concluded there was a single "primary matter". He reasoned if you constantly divide objects into small pieces, you can make a very tiny piece that can no longer be cut. He called that as an atom and he defined atom as matter consists of invisible particles. Also, he stated that atoms are indestructible, unchangeable, and homogenous. This was a very notable theory that tried to explain the whole physical world using a small number of ideas. Lived- 460-370 BC Nation- Greece State atomic model in 442 BC PART 1 01/ 7 Democritus John Dalton J.J. Thomson Robert A. Millikan Eugen Goldstein James Chadwick Ernest Rutherford John Dalton John Dalton was English chemistry and teacher and he researched the matter which is the smallest particles in the universe. He stated all matters consist of indivisible particles and he named each small particle. Atoms of the same element all have the same mass and he wanted to symbol each of them to distinguish. He imagined that the shape of the atom will be a sphere. We cannot destruct and remain its identity through chemical reactions. The compound is created by combine atoms in small whole-number ratios. Lived- 1766-1844 Nation- England State atomic model in 1803 PART 2 02/ 7 Democritus John Dalton J.J. Thomson Robert A. Millikan Eugen Goldstein James Chadwick Ernest Rutherford J.J. Thomson J.J. Thomson was an English scientist and his model was known as the "Plum Pudding Model” The atom was a sphere and filled with a positively charged fluid, known as the “pudding”. It was negative charge electrons scattered in this liquid, and these were pudding's "plums." He claims that positive fluids held the negatively charged electrons because of its electrical forces. After Thomson discovered the electron, the atom was not an indivisible particle. During a discharge tube experiment, Lived- December 18, 1856 - August 30, 1940 Nation- England State atomic model in 1904 PART 3 03/ 7 Democritus John Dalton J.J. Thomson Robert A. Millikan Eugen Goldstein James Chadwick Ernest Rutherford Robert A. Millikan Robert Millikan was an American physicist and used an oil-drop experiment to determine the electric charge carried by a single electron. He concluded that the charge of an electron is 1.59(10^19) C. It is very similar to today’s value. He also found the quite accurate value of mass which is 9.1(10^-28) g. Robert A. Millikan Lived- March 22, 1868 - December 19, 1953 Nation- U.S. PART 4 04/ 7 Democritus John Dalton J.J. Thomson Robert A. Millikan Eugen Goldstein James Chadwick Ernest Rutherford Eugen Goldstein Eugen Goldstein is a German physician who discovered protons which have an equal and opposite charge as an electron. He found a cathode ray which is another ray moving in the opposite direction from the positive to the negative. He also judged that the mass of the proton was 1,840 times heavier than that of the electron. Eugen Goldstein Lived- 1850-1930 Nation- German PART 5 05/ 7 Democritus John Dalton J.J. Thomson Robert A. Millikan Eugen Goldstein James Chadwick Ernest Rutherford James Chadwick James Chadwick is a person who discovered neutrons using his atomic theory. The protons were large and lumped together in the nucleus, and the electrons circled the nucleus in a circle. Neutrons were very difficult to find because they did not push out protons when they were in atoms. Because of the discovery of neutrons, models of the appropriate atoms have become available to chemists. Lived- 20 October 1891 – 24 July 1974 Nation- England State atomic model in 1932 PART 6 06/ 7 Democritus John Dalton J.J. Thomson Robert A. Millikan Eugen Goldstein James Chadwick Ernest Rutherford Ernest Rutherford Ernest Rutherford is a New Zealand-born British physicist and he postulated the nuclear structure of the atom. He imagined the atom as a miniature solar system, with electrons orbiting around a massive nucleus, and like most empty spaces, the nucleus occupies only a tiny part of atoms. Lived August 30, 1871- October 19, 1937 Nation- New Zealand-born British State atomic model in 1911 PART 7 07/ 7 FINISH FINISH FINISH FINISHe FINISH FINISH FINISH Luke Noh Chris Chang First page atom icon - Icons made by <a href="https://www.flaticon.com/authors/freepik" title="Freepik">Freepik</a> from <a href="https://www.flaticon.com/" title="Flaticon"> www.flaticon.com</a> Democritus’ concept of the atom. (n.d.). [Ilustration]. Sutori. https://www.sutori.com/item/democritus-did-a-very-simple-experiment-he-took-a-seashell-and-broke-it-inhalf-095c Dalton’s atomic theory. (n.d.). [Illustration]. Pinterest. https://www.pinterest.co.kr/pin/790592909555654907/?autologin=true [Illustration about experiment]. (n.d.). Wikipedia. https://en.wikipedia.org/wiki/Discovery_of_the_neutron J.J Thomson. (n.d.). [Illustration]. Khanacademy. https://www.khanacademy.org/science/ap-chemistry/electronic-structure-of-atoms-ap/history-of-atomic-structureap/a/discovery-of-the-electron-and-nucleus Robert’s oil drop experiment. (n.d.). [Illustration]. Lumen. https://courses.lumenlearning.com/introchem/chapter/millikans-oil-drop-experiment/ Anode Rays Discharge tube. (n.d.). [Illustration]. Topper Learning. https://www.topperlearning.com/answer/explain-the-formation-of-protons-from-gas-discharge-tube-asstated-by-goldstein/3xzq3b722 Rutherford’s gold hil experiment. (n.d.). [Illustration]. Scratic Q&A. https://socratic.org/questions/how-did-rutherford-s-gold-foil-experiment-differ-from-his-expectations Gregersen, Erik. “Rutherford Model.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 1998, www.britannica.com/science/Rutherford-model. The History of the Atom, thehistoryoftheatom.weebly.com/. Dowd, Brian. “Eugene Goldstein (1885) - Atomic Structure.” Google Sites, sites.google.com/site/structureatomic/460-bc-and-the-1800-s/eugene-goldstein-1885. Lee, Cindy. “1909 Robert Millikan - Atomic Structure.” Google Sites, 2013, sites.google.com/site/atomicstructure11/history/millikan. Thank you! PART 7