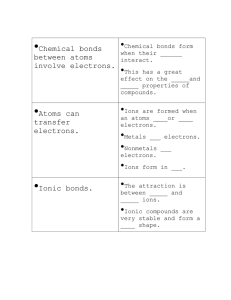
Chemistry C2 1.1-2.5 Atoms are made of protons, neutrons and electrons. Protons have a positive + charge and electrons have a negative – charge. Atoms are arranged in the periodic table in order of their atomic number. Neutrons have no electric charge so are neutral. Atomic Structure The element Lithium has a symbol of Li. 3 electrons (atomic number) and an atomic mass of 7 The electrons in an atom are arranged in shells. Atoms with the same number of electrons in their outer shell belong in the same group of the periodic table. The number of electrons in the outer shell of an atom determines the way that the atom behaves in chemical reactions. Covalent Bonding Ionic Bonding Ionic compounds are held together by strong forces between the oppositely charged ions. This is called ionic bonding. Group 2 & Group 6 elements can form ionic compounds. This happens between a metal and non-metal. These form giant structures of ions in a lattice. This takes place between non-metals. Covalent bonds are formed when atoms share electrons. Many substances contain covalent bonds consist of molecules, but some have giant covalent structures. The electrons are shared so there is an outer shell of 8 electrons. Chemical Bonding Elements react to form compounds by gaining or losing electrons or by sharing electrons. The elements in Group 1 react with the elements in Group 7 because group 1 needs to lose an electron from its outer shell and group 7 need to gain an electron to have a full outer shell. This can be shown by using dot and cross diagrams. Remember: An outer shell which does not have a full shell of 8 electrons is unstable. So all elements are looking to become stable. Metallic Bonding The atoms (or ions) in metals are arranged in regular layers. The positive ions in metals are held together by electrons from the outer shell of each metal atom. These form giant structures. The diagram on the left shows this. Ionic compounds – it takes a lot of energy to break the bonds which hold a giant ionic lattice together. Very high melting points and are solid at room temperatures. They conduct electricity when melt or dissolve them in water because their ions can then move freely. Chemistry C2 3.1-4.6 Equations & calculations Mass Numbers Chemical equations tell us the number of moles of substances in the chemical reaction. These can be used to calculate the masses of reactants and products in a chemical reaction from the masses of one mole of each of the substances involved in the reaction. The relative mass of protons and neutrons is 1. The mass number of an atom tells you the total number of protons and neutrons in its nucleus. Isotopes are atoms of the same element with different numbers of neutrons. Work out the relative formula mass of a compound from the relative atomic masses of the elements in it. Yield: of a chemical reaction describes how much product is made. Making Ammonia – the Haber process Ammonia is important for making other chemicals, e.g. fertilisers. It is made from nitrogen and hydrogen in the Haber process. This is where the process is carried out under conditions which are chosen to give a reasonable yield of ammonia very quickly. Any unused nitrogen and hydrogen are recycled in the Haber process. See diagram of Haber process. Reversible reactions In this reaction the products of the reaction can react to make the original reactants. The rate of the reaction in each direction is controlled by Enzymes. Collision theory- as particles react they begin to collide, the faster they collide the more energy is made. The rate of a chemical reaction increases if the surface area of any solid reactants is increased. Effects of temperature, concentration & increasing pressures – faster reactions, as particles collide more often and have more energy. A Catalyst – speeds up the rate of a chemical reaction. It is not used up during a chemical reaction. We can measure the rate of reaction by following the rate at which reactants are used up. Chemistry C2 5.1 -6.4 Thermal decomposition These reactions are also endothermic e.g. calcium carbonate to form calcium oxide and carbon dioxide. Exothermic & Endothermic Reactions Energy may be transferred to or from the reacting substances in a chemical reaction. A reaction that produces heat energy is called an exothermic reaction. A reaction where energy is taken to the reactants is called an endothermic reaction. Electrolysis the basics This involves splitting up a substance using electricity. Ionic substances can be electrolysed when they are molten or in solution. Positive ions move to the negative electrode (cathode) and negative ions move to the positive electrode (anode). Changes at the electrodes In electrolysis the ions move towards the oppositely charged electrodes. The negative ions are oxidised while positive ions are reduced. Electrolysing brine This gives us 3 products – chlorine gas, hydrogen gas and sodium hydroxide solution. Reactions where reduction and oxidation happen are called REDOX reactions. When electrolysis happens in water, the less reactive element is usually produced at an electrode. Purifying Copper Copper extracted from its ore contains impurities of gold & silver. Copper is purified by electrolysis to remove these impurities. Energy & reversible reactions In any reversible reaction, the amount of energy released when the reaction goes in one direction is exactly equal to the energy absorbed when the reaction goes in the opposite direction. Chemistry C2 7.1 – 7.3 Acids & Alkalis Acids are substances which produce H+ ions when we add them to water. Bases are substances that will neutralise acids. An alkali produce OH- ions when we add them to water. The pH scale is used to show how acidic or alkaline a solution is. Making salts from solutions An indicator is needed when we produce a salt by reacting an alkali with an acid to make a soluble salt. Insoluble salts can be made by reacting 2 solutions to produce a precipitate. Precipitation is an important way of removing some substances from wastewater. Making salts from metals or bases An acid reacted with an alkali makes a neutralisation reaction. The reaction between an acid and a base produces a salt and water. Salts can also be made by reacting a metal with an acid. This reaction also produces hydrogen gas as well as a salt.