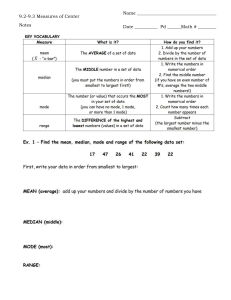
FLOW CYTOMETRY I had dinner with my friend Celine and mentioned that I spent my day on the FACS machine. She was really surprised that a professor at McGill can spend an entire day sending FAX to people! I had to explain that FACS is not FAX but stands for fluorescenceactivated cell sorting or flow cytometry. What is flow cytometry? It has something to do with cells? Tell me more about it. Let see if I did a good job in explaining to my friend Celine, who has a good scientific background, what is flow cytometry and whether you could do better! Celine’s Questions What is flow cytometry? How does it work? What are the basic components of a flow cytometer? How analysis of individual cells is achieved? Which type of information can you obtained from this technology? How cell size is measured? How cell granularity/complexity is measured? How expression of various molecules by the cells is measured? Give me an example of data for the expression of a given molecule. Which conclusions can you make from these data? Is it possible to detect more than one molecule? What is compensation? How compensation is set? What are the consequences of bad compensation? Bold red questions = in-class problem What is Flow Cytometry? Flow cytometry…. How does it work? individual cells in a fluid medium pass throug a laser beam and the light that emerges from the cell is captured and transformed into quatititative data What are the basic components of a flow cytometer? the fluidifc system, which carried and delivers indicial cells to the lught source the laserss which is the light source How analysis of individual cells is achieved? This is accomplished by hydrodynamic focusing in which cells in liquid suspension are injected into a flowing stream of sheath fluid that is compressed, forcing the cell into the center of the stream that has roughly one cell in diameter. Which type of information can you obtained from this technology? • Cell size • Cell granulocyty/complexity • Expression of several different cell surface and/or intracellular molecules How cell size is measured? As a cell passes through the laser beam it will diffract or scatter light in all direction. It is possible to measure the amount of light scattered in the forward direction as a cell passes through the laser beam. Small cells scatter a small amount of light in the forward direction while larger cells scatter a larger amount of light. The amount of light scattered in the forward direction (the forward scatter or FSC) is thus roughly proportional to the size of the cell. How cell size is measured? Relative cell number The amount of forward scattered light is quantified by a detector that converts light intensity into an electronic signal that is digitized and sent to a computer. Small cells produce a small FSC signal and larger cells generate a higher FSC signal. If a graph is plotted for the intensity of the FSC signal for each cell versus the relative cell number in a sample, small cells appear on the left of the FSC axis while larger cells appear further on the right. It is thus possible to have a graphical representation of the size of the cells in a given sample (this is called an histogram). Cells from 0.2 to 50 um can be easily detected by flow cytometry. FSC FSC How cell granularity/complexity is measured? Light scattering to the side (90°), is caused by granularity and complexity inside the cell. This side-scattered light (SSC) is focused through a lens system and is collected by a separate detector. Two-dimensional representations It is possible to have a graphical 2-D representation of combined parameters for each cell. One example of such 2-D representation is called a dot plot. Here is an example from human blood (in which red blood cells have been partially lysed) A two-dimensional diagram where the SSC signals are plotted against FSC signals for individual cells reveals the presence of 3 cell types (each dot corresponds to a cell): Lymphocytes which are small cells possessing low internal complexity; monocytes which are medium-sized cells with slightly more internal complexity, and granulocytes which are large cells that have a lot of internal complexity. The cells in the red gate are aggregated red blood cells and cell debris. 4000 SSC 3000 2000 1000 0 0 1000 2000 FSC 3000 4000 How expression of a given molecule by the cells is measured? Cell surface and/or intracellular molecules are detected by specific Abs labeled with fluorescent dyes (fluorophores). The cells are labeled with these Abs and when they pass through the laser beam the dye molecules bound to the cells are excited and emit fluorescence which is detected by photomultiplier tubes. The amount of fluorescence emission of a given dye is proportional to the number of labeled Ab bound to the cell. Thus, fluorescence emissions give information on the binding of the labeled Abs and hence on the expression of proteins by each cell. Give me an example of data for the expression of a given molecule Cells expressing low levels of the molecule produce a small fluorescent signal and cells expressing higher levels of the molecule generate a higher fluorescent signal. If a graph is plotted for the intensity of the fluorescent signal for each cell versus the relative cell number in a sample (an histogram), low expressing cells appear on the left of the fluorescent axis ( x axis) while higher expressing cells appear further on the right. M1 Unstained cells (autofluorescence) M1 M2 100 101 102 iso gran b 103 104 100 101 M2 102 gran b 103 M1 104 100 population is more on the right in the first 2 graphs M1 is the region of negaative M2 is the regions of positivity - in the 2 histograms, the one at the right produces less fluorescnece (the peak in M2 is lower 101 M2 102 Perforin 103 104 Which conclusions can you make from these data? 0 M1 200 400 600 800 1000 FSC Based on the histogram of the unstained cells, I can put markers (M2) and determine the percentage of cells that express the molecule of interest. I can do this on different populations. Based on the SSC/FSC profile of my sample I can choose to analyze a specific population (gated population) for the expression of the molecule (red or blue population). I can determine the mean expression level of the molecule between different populations (red versus blue population). Unstained cells (autofluorescence) M1 M2 100 File: 150208.005 Marker All M1 M2 fluorescence by the population in the blue circle - larger more complex cells — less expression of molecule 101 102 iso gran b Events % Gated 7950 100.00 7874 99.04 92 1.16 103 100 104 101 M2 102 gran b Mean Geo Mean Median 5.16 4.79 4.53 5.03 4.73 4.53 17.43 16.44 14.86 103 M1 104 100 File: 150208.004 Marker All M1 M2 Events % Gated 8010 100.00 50 0.62 7965 99.44 102 Perforin Events % Gated 8032 100.00 1490 18.55 6676 83.12 File: 150208.006 Marker All M1 M2 101 M2 Mean Geo Mean Median 83.28 59.83 50.48 10.13 9.79 10.75 83.69 60.46 50.48 103 104 Mean Geo Mean Median 25.46 20.91 19.81 9.49 9.10 10.00 28.76 24.91 22.88 Can you draw a 2D representation of these data? 100 101 102 Foxp3 103 104 Is it possible to detect more than one molecule? It is now possible to perform 21-color analysis (meaning that it is possible to use the expression of 21 different cell- surface and/or intracellular proteins to characterize various cell subsets in a given sample). Flow cytometry is a very powerful tool. When using more than one fluorophore, it is critical to compensate. What is compensation? What is compensation? What is compensation? What is compensation? How to set compensation? As an example we will perform a stain of lymph nodes cells for CD8 and CD4 T cells, using a CD4-PE mAb and a CD8-FITC mAb. In addition to the experimental stain (cells + anti-CD4-PE + anti-CD8-FITC), cells are also stained with individual fluorophores (cells + anti-CD4-PE only and cells + anti-CD8-FITC only). Unstained cells are also necessary. Unstained cells allow for setting forward scatter and side scatter voltages so that the cell population is clearly delineated. Unstained cells are also used to set the voltages for fluorescence channels in such a way that the entirely unstained population is completely off the lower axis for every parameter being measured. (i.e. all the cells should appear in the lower left corner of the dot plot CD4 vs CD8) Cells stained with only the CD8-FITC mAb are examined. The bright FITC positive cells show an appreciable signal in the FL2 detector. The FL2-%FL1 compensation control is then gradually increased until the median FL2 channel number of the FITC-bright cells is the same as the median FL2 channel number of the FITC-neg cells (the centers of the two populations are equal). Compensation is correctly set when the median of the negative population is equal to the median of the positive population in the spillover channel. For example, if using a positive FITC-stained sample to apply compensation to the PE channel, the median of the negative and the FITC-positive populations should be equal in the PE channel. Repeat for all channels into which FITC leaks (if doing more than 2 color analysis). Repeat for all single color controls. Cells stained with only the CD4-PE mAb are examined. The bright PE positive cells show a signal in the FL1 detector. The FL1%FL2 compensation control is then gradually increased until the median FL1 channel number of the PE bright cells is the same as the median FL1 channel number of the PE-neg cells (the centers of the two populations are equal). Compensation is correctly set when the median of the negative population is equal to the median of the positive population in the spillover channel. For example, if using a positive PE-stained sample to apply compensation to the FITC channel, the median of the negative and the PE-positive populations should be equal in the FITC channel. Repeat for all channels into which PE leaks if doing more than 2 color analysis. What are the consequences of bad compensation? Correctly compensated Under compensated Over compensated