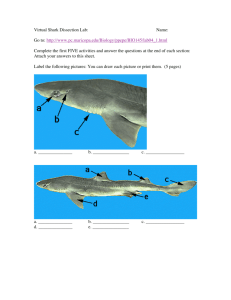
Ann Surg Oncol (2017) 24:2189–2198 DOI 10.1245/s10434-016-5691-4 ORIGINAL ARTICLE – ENDOCRINE TUMORS The Effect of Prophylactic Central Neck Dissection on Locoregional Recurrence in Papillary Thyroid Cancer After Total Thyroidectomy: A Systematic Review and Meta-Analysis pCND for the Locoregional Recurrence of Papillary Thyroid Cancer Wenjing Zhao, MD, Lei You, MD, Xianming Hou, MD, Shaobo Chen, MD, Xiaoxia Ren, PhD, Ge Chen, MD, and Yupei Zhao, MD Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, China ABSTRACT Background. The use of prophylactic central neck dissection (pCND) for papillary thyroid cancer (PTC) without clinical evidence of nodal metastasis (cN0) remains controversial. This study was designed to examine whether pCND for PTC affected locoregional recurrence (LRR). Methods. A systematic review was performed to compare the LRR between patients with PTC who underwent total thyroidectomy (TT) and pCND and those who underwent TT alone. The primary outcome was LRR. Other outcomes, including postoperative radioiodine (RAI) ablation and surgically related complications, were evaluated. A metaanalysis was performed using the random-effects model. Results. We included 17 studies, which comprised 4437 patients. Patients in the TT?pCND group had a significantly reduced risk of LRR (risk ratio [RR] = 0.66; 95% confidence interval [CI]: 0.49–0.90; P = 0.008). The LRR in the central neck compartment (RR = 0.35; 95% CI 0.18–0.68; P = 0.002) was significantly lower in the Wenjing Zhao and Lei You contributed equally to this article. Electronic supplementary material The online version of this article (doi:10.1245/s10434-016-5691-4) contains supplementary material, which is available to authorized users. Ó Society of Surgical Oncology 2016 First Received: 28 June 2016; Published Online: 2 December 2016 G. Chen, MD e-mail: pumchchenge@163.com Y. Zhao, MD e-mail: zhao8028@263.net TT?pCND group, whereas the LRR in the lateral neck compartment was similar between the two groups. Compared with the TT alone group, patients in the TT?pCND group tended to receive higher RAI (74.6% vs. 59.9%) and experience temporary hypocalcemia (odds ratio [OR] = 2.37; 95% CI 1.89–2.96; P \ 0.00001), permanent hypocalcemia (OR = 1.93; 95% CI 1.05–3.57; P = 0.03), and increased overall morbidity (OR = 2.56; 95% CI 1.75–3.74; P \ 0.00001). Conclusions. This meta-analysis suggested that although pCND reduced the LRR in PTC—specifically in the central neck compartment—it was accompanied by an increased rate of postoperative hypocalcemia. However, the evidence is limited and randomized, controlled trials are needed to clarify this role further. Thyroid cancer, the most common endocrine malignancy, continues to be the most rapidly increasing cancer ([5% per year in both men and women) with an estimated 64,300 new cases diagnosed in the United States in 2016.1 Papillary thyroid cancer (PTC) comprises the majority (80–85%) of these cases, and the prognosis for treated PTC patients is excellent with 10-year survival rates exceeding 90%. Despite having an excellent prognosis, PTC patients commonly experience lymph node metastases (20–50%), mostly in the central compartment of the neck (level VI) comprising the prelaryngeal (Delphian), pretracheal, and paratracheal nodal basins.2 Lymph node metastases are associated with an increased rate of locoregional recurrence (LRR).3,4 Although therapeutic central neck dissection (CND) is indicated in PTC patients with clinically nodal-positive disease (cN1), it 2190 remains controversial whether prophylactic central neck dissection (pCND) should be performed in patients with no clinical evidence of nodal metastasis (cN0).5,6 Currently, there was insufficient evidence to draw a conclusion about the effect of combining total thyroidectomy (TT) with pCND on the LRR compared with TT alone. Some studies favored pCND, because it could prevent regional recurrence and may be associated with lower postsurgical thyroglobulin (Tg) levels, a higher dose of radioactive iodine (RAI) due to pathologic upstaging, and a lower complication rate after the first operation.7–9 However, other studies reported that although pCND decreased postoperative Tg levels, it is not helpful in decreasing short-term LRR in patients (cN0).9–12 Furthermore, this procedure increases the risk of morbidity regarding injury to the parathyroid glands and recurrent laryngeal nerves.7,13–15 Therefore, we should weigh the potential advantages of pCND against the risk of complications. This controversy is partly due to a lack of high quality evidence demonstrating any legitimate benefits of pCND in reducing LRR. Given the low rates of recurrence and morbidity after thyroidectomy, a recent study estimated that 5840 patients in a prospective, randomized, controlled trial would be necessary to have sufficient statistical power to detect a 25% reduction in the recurrence risk.16 Although five relevant meta-analyses were conducted through 2013, three were not strictly consistent with our research subject; two included patients who either had benign disease or experienced therapeutic CND, and one excluded patients with papillary microcarcinoma.13,17–20 The remaining two studies showed different conclusions.13,19 Zetoune et al. found that pCND does not greatly reduce LRR, whereas Lang et al. showed that pCND may create a 35% reduction in the risk of LRR.13,19 Because the literature has been updated in the past 3 years, we performed a systematic review and metaanalysis to investigate the effect of pCND on the LRR with the current largest sample size. METHODS Search Strategy This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.21 Studies were identified using a literature search of English articles in the PubMed, EMBASE, and Cochrane Library databases published through November 27, 2015. The search parameters were as follows: (papillary thyroid carcinoma) AND thyroidectomy AND [(lymph node dissection) OR (neck dissection) OR (central neck dissection) OR (level VI neck dissection) OR (level 6 neck dissection)]. The reference lists of W. Zhao et al. previous meta-analyses also were reviewed to identify additional potentially relevant articles. Two authors searched articles and independently reviewed all retrieved studies. Disagreements between the two investigators were resolved by consensus with a third investigator. Study Selection The inclusion criteria were as follows: (1) a prospective or retrospective study design; (2) a PTC diagnosis with cN0 as determined by preoperative imaging and intraoperative examination; (3) two-arm studies comparing TT with pCND (without lateral compartment dissection) to TT alone; (4) sample size per arm [10 patients; (5) reporting of the number of LRR and the mean follow-up period (in months) in each study arm; and (6) patient age C18 years. Accordingly, the following exclusion criteria were also used: (1) clinical evidence of lymph node metastases by preoperative imaging and intraoperative examination; (2) patient history of hemithyroidectomy with pCND or simultaneous pCND and prophylactic lateral neck dissection; (3) lack of recurrence data; or (4) the article was published as a case report, review article, letter to the editor, editorial, or conference abstract. Data Extraction Two researchers independently extracted the following data from the selected studies: the first author’s last name, year of publication, country of origin, study design, preoperative nodal assessment, method of selection for pCND, tumor characteristics, number of patients who underwent TT?pCND or TT, extent of pCND (unilateral vs. bilateral), number of harvested normal and metastatic central LNs, upgrade rate from stage 1/2 to stage 3 because of nodal status, number of patients receiving RAI treatment, number of LRRs, site of locoregional recurrence, and postoperative complications. The quality of each study was evaluated based on the Recommendation of the Cochrane Handbook for Systematic Reviews of Inventions [Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011)]. Statistical Analysis Review Manager 5.2 software was used to analyze the outcomes. The Q test was performed to assess the heterogeneity among studies with the I2 index as an indicator of true heterogeneity. Statistical heterogeneity was considered high when I2 [ 50%. All results were aggregated and analyzed using a random-effects model. The LRR was assessed by the risk ratio (RR), whereas the odds ratio (OR) was examined for all other outcomes. R 3.2.3 software was pCND for the Locoregional Recurrence of Papillary Thyroid Cancer 2191 used to conduct sensitivity analysis to investigate potential studies that influence the reliability of results. P value B0.05 was considered to be statistically significant, and the 95% CI was set to measure the effects. Kingdom together.7–9,11,12,14,15,22–31 All studies featured ultrasonography as the standard imaging procedure for preoperative nodal assessment. With respect to the extent of pCND, 6 of the 17 studies used bilateral pCND, 5 used unilateral or bilateral, 1 used unilateral, and 5 studies did not classify the pCND.7–9,11,12,14,15,22–31 Fifteen of 17 studies compared age, sex ratio, tumor size, extrathyroidal extension, and multifocality between the two groups. For bilateral pCND, the mean number of central LNM harvested ranged from 5.6 to 8.8, with the incidence of central LNM ranging from 33.3 to 82.4% for the TT?pCND group. With the high incidence of central LNM, the percentage of patients older than 45 years of age undergoing tumor upstaging ranged from 3.2 to 34.7%. RESULTS Literatures Search Our search strategy yielded 1231 abstracts from the three databases. After excluding duplicate articles, two reviewers independently reviewed 986 abstracts, of which 46 articles were reviewed in full text. By monitoring the reference lists, we identified 4 additional articles.11,12,22,23 Of the 50 articles, 33 were subsequently excluded from the meta-analysis for various reasons, and 17 articles (3 prospective studies and 16 retrospective studies) involving 4437 thyroid papillary cancer cases (2468 in the TT group and 1969 in the TT?pCND group) were included.7–9,11,12,14,15,22–31 The description of our literature search is illustrated in Fig. 1. The characteristics of these studies are presented in Table 1. The publication years ranged from 2007 to 2015; five studies were conducted in the United States, five in Italy, four in Korea, one in China, one in Poland, and one study in Australia, the United States, and the United Radioiodine (RAI) therapy was reported in 14 studies (Table 1).7–9,11,22,23,25–30 The mean postoperative RAI rate was 74.6% in the TT?pCND group and 59.9% in the TT group, indicating a higher RAI ablation therapy rate for TT?pCND (OR = 1.20, 95% CI 1.04–1.39). Seven of the 14 studies reported the dose for RAI, 3 administered a greater I131 ablation dose to those with nodal involvement, and the remaining 4 studies gave the same dose to both groups.7,9,14,15,25,29,30 Locoregional Recurrence Literature search in databases; n=1231 Pubmed: n=663 Embase: n=557 Cochrane Library: n=11 Duplicates: n=245 Titles and abstracts reviewed: n=986 Non-relevent: n=940 Articles for further review: n=46 Radioiodine Ablation Therapy Articles from reference list: n=4 Articles reviewed in full text: n=50 Data not available: n=16 Therapeutic neck lymph node dissection: n=7 Not tatal thyroidectomy: n=5 Reviews: 5 Articles included for meta-analysis: n=17 FIG. 1 Search strategy and study selection The results of the meta-analysis showed a significantly reduced locoregional recurrence (LRR) rate for the TT?pCND group compared with the TT group (4.6% vs. 6.9%, RR = 0.66; 95% CI 0.49–0.90; P = 0.008; I2 = 19%; Table 2). We evaluated the central and lateral site of lymph node recurrence in 12 studies (Fig. 2), which included 3396 patients (1446 received TT?pCND; 1950 received TT). A total of 7572 (2.2%) patients showed recurrence in the central lymph node region and 105 (3.1%) patients in the lateral lymph node region. Patients in the TT?pCND group experienced a reduced risk of recurrence to the central lymph node region than that in the TT group (1.1% vs. 3.4%, RR = 0.35; 95% CI 0.18–0.68; P = 0.002; I2 = 13%). However, the rate of recurrence in the lateral lymph node region was similar between both groups (3.3% vs. 3.2%, respectively). Operative Outcomes As shown in Fig. 3, patients in the TT?pCND group had a significantly increased risk of postoperative hypocalcemia rate, including temporary hypocalcemia (28.7% vs. 17.5%, OR = 2.37, 95% CI 1.89–2.96; P \ 0.00001, I2 = 27%) and permanent hypocalcemia USA Italy USA USA Australia,USA and UK Zuniga,23 2009 Costa,28 2009 Moo,29 2010 Hughes,30 2010 Popadich,31 2011 Retro Retro Retro Retro Retro Retro Korea Poland Barczynski,7 2013 Italy So,9 2012 Raffaelli, 2012 24 China Retro Retro Pro Retro Italy Perrino,12 2009 Retro Lang,8 2012 Korea Choi,11 2008 Retro Retro Korea Roh,27 2007 Study design Wang,22 2012 USA Country Study Bi Bi Uni and Bi Uni NA Uni and Bi Bi Bi Uni and Bi NA Uni and Bi NA Bi 640 232 186 185 86 606 143 81 244 266 251 101 113 Extent of Patients pCND (N) TABLE 1 Clinical characteristics of the included studies 41.5 43.2 49.8 TT alone NA TT alone 282 NA TT?pCND 358 113 49.2 TT?pCND 119 62 TT alone 50 42.7 103 TT alone 52 NA TT?pCND 124 82 37 TT alone TT?pCND 49 NA 48 TT?pCND 20.4 NA 60/ NA 222 75/ NA 283 16/97 6.2 21/98 6.6 13/49 12.1 24/ 12.9 100 22/81 10 NA 6.7 NA NA 1.5 9.6 0 5 NA 6a 0.35 6.8 0a 6.0a 1.7 8.8 NA NA NA NA NA NA NA NA NA 5.6 NA NA NA 37.0 9.7 35.5 4.9 54.9 NA 40.8 6.0 49.0 9.2 61.5 16.7 33.3 5.9 46.8 NA 82.4 NA NA NA 37.5 NA 62.2 NA NA NA NA NA NA NA 17.1 NA 34.7 1.0 10.0 NA 28.6 NA NA NA NA NA NA NA NA NA NA NA NA Mean no. of central Incidence of Upstaged to LNS excised central LNM (%) stage III (%) 14a 18/64 15 NA NA 81/ 22.3 266 52/ 22.7 205 16/49 20a 4/32 10/35 14.2 24/94 15 26/ 17 100 13/ NA 117 41.2a TT alone 347 NA NA 10/ NA 126 NA NA 17/61 19a 44 65 TT alone 6.8 22 NA 11/42 7.3 6/42 9/64 NA 46.8a 49.2 TT?pCND 259 78 36 TT TT?pCND 45 TT?pCND 45.7 52 TT alone 118 46 TT?pCND 126 130 TT alone NA 42.9 159 TT alone NA 48 52 48.5 NA Mean age Sex Mean tumor (years) (M:F) size (mm) TT?pCND 136 92 53 TT?pCND TT alone 73 48 TT alone TT?pCND 40 Patients (N) TT?pCND Group 79 231 92 101 NA NA 63 62 12 35 NA NA 56 72 26 31 62 87 55 79 NA NA 53 48 73 40 No. of RAI ablation 2192 W. Zhao et al. 2193 (4.1% vs. 2.3%, OR = 1.93; 95% CI 1.05–3.57; P = 0.03, I2 = 30%) than patients in the TT group. However, the definition of permanent hypocalcemia varied among the studies. Nine studies defined permanent hypocalcemia as hypocalcemia and/or the need for continued calcium supplementation for longer than 6 months, whereas longer than 12 months was the definition in two studies.8,9,11,14,15,22,25,26,29–31 When including the nine studies for a consistent definition, the TT?pCND group had significantly higher temporary (27.4% vs. 19.6%, OR = 2.44, 95% CI 1.75–3.38; P \ 0.00001, I2 = 40%) and permanent hypocalcemia rates (6.3% vs. 2.6%, OR = 2.36; 95% CI 1.10–5.06; P = 0.03; I2 = 35%) compared with those of the TT group. Regarding other operative outcomes, the results showed that patients in both groups had a similar risk for developing temporary (OR = 1.22, 95% CI 0.85–1.75; P = 0.27, I2 = 0) and permanent RLN injury (OR = 1.17, 95% CI 0.56–2.44; P = 0.68, I2 = 20%), hematomas (OR = 1.19, 95% CI 0.54–2.16; P = 0.67, I2 = 0%), and wound infection/seroma (OR = 0.85, 95% CI 0.37–1.92; P = 0.69, I2 = 0%). Furthermore, only two studies compared the operating times; therefore, we did not pool these data.24,26 Regarding overall complications, the result of meta-analysis indicates that the TT?pCND group has a significantly increased overall morbidity rate (OR = 2.56; 95% CI 1.75–3.74; P \ 0.00001; I2 = 79%; Supplementary Fig. S1) but was similar after excluding temporary hypocalcemia (OR = 1.14; 95% CI 0.84–1.55; P = 0.40; I2 = 27%; Supplementary Fig. S2). Sensitivity Analyses Sensitivity analyses performed demonstrated that the findings of all the available studies were robust (Supplementary Table S1). Medians were provided DISCUSSION a Retro retrospective study, Pro prospective study, pCND prophylactic central neck dissection, TT total thyroidectomy, Uni unilateral, Bi bilateral, LN lymph node, LNM lymph node metastasis, RAI radioactive iodine, NA not available 74 NA NA NA 51.6 TT alone 104 16/88 16 112 NA NA NA 52.3 Korea Lee,26 2015 Pro NA 257 TT?pCND 153 30/ 17 123 93 88 NA 3.2 46.2 6.8 NA NA 25/93 16 21/88 16 45.7 43.5 93 88 TT?pCND 181 NA Italy Viola,25 2015 Pro 580 Uni and Bi Retro USA De Carvalho,14 2015 TT alone 212 NA NA NA 45.2 478 TT alone 55/ 10.2 423 58 NA NA 6.8 41.2 TT?pCND 102 15/87 14.8 194 65 NA NA NA NA 7.5 NA 48/ 16.13 172 53.07 12/53 17.27 46.36 65 220 TT?pCND TT alone 285 Bi Italy Calò,15 2014 Retro Country Study TABLE 1 continued Study design Extent of Patients pCND (N) Group Patients (N) Mean age Sex Mean tumor (years) (M:F) size (mm) Mean no. of central Incidence of Upstaged to LNS excised central LNM (%) stage III (%) No. of RAI ablation pCND for the Locoregional Recurrence of Papillary Thyroid Cancer One of the most controversial issues in the treatment of papillary thyroid cancer is the management of CND. Although therapeutic CND is recommended for patients with cN1 PTC, the necessity of pCND in clinically nodenegative PTC is controversial.32,33 Many studies have investigated the potential advantages of TT?pCND compared with TT in decreasing postoperative Tg, elevating the tumor stage, and reducing the LRR. However, it remains unclear whether extended TT is beneficial in decreasing the LRR. Furthermore, we should weight the potential accompanying disadvantages of postoperative complications and overuse of adjuvant radioactive iodine therapy due to upstaging the tumor with no effect in reducing the LRR.34 2194 W. Zhao et al. TABLE 2 Details for locoregional recurrence of included studies Study Group Roh,27 2007 TT?pCND 51 0 (0) 0 0 TT alone 53 3 (4.1) 3 2 TT?pCND 24.4 1 (2.1) 0 1 TT alone 24.4 2 (3.8) 0 2 TT?pCND 82.8 19 (14.0) NA NA TT alone 82.8 26 (20.0) NA NA NA Choi,11 2008 Zuniga,23 2009b Perrino,12 2009b Mean follow-up (months) LRR, n (%) CNR LNR TT?pCND 69.2 5 (5.4) NA TT alone 69.2 22 (13.8) NA NA Costa,28 2009 TT?pCND 47 8 (6.3) 4 5 TT alone 64 9 (7.7) 4 5 Moo,29 2010 TT?pCND TT alone group 37.2 37.2 2 (4.4) 6 (16.7) 0 2 1 4 Hughes,30 2010 TT?pCND 19.1a 4 (5.1) 2 2 TT alone 27.5a 2 (3.1) 2 0 31 Popadich, 2011 So,9 2012 Raffaelli,24 2012 Lang,8 2012 Wang,22 2012 TT?pCND 32 13 (5.0) 4 11 TT alone 50 29 (8.4) 22 21 TT?pCND 44.7 2 (1.7) 1 2 TT alone 45.4 4 (3.5) 3 1 TT?pCND 25 1 (0.8) 1 0 TT alone 25.5 0 (0) 0 0 TT?pCND 25.5a 3 (3.7) 0 3 TT alone 27.1a 3 (2.9) 0 3 TT?pCND 21a 0 (0) 0 0 TT alone 21a 0 (0) 0 0 Barczynski,7 2013 TT?pCND 126.4 15 (4.2) 2 13 128.8 37 (13.1) 22 15 Calò,15 2014 TT?pCND TT alone 2 (3.1) 4 (1.8) 0 1 2 4 Viola,25 2015b TT?pCND 59.4 7 (7.5) NA NA TT alone 59.4 7 (8.0) NA NA TT alone Lee,26 2015b De Carvalho,14 2015 100a 100a TT?pCND 55.2 5 (3.3) NA NA TT alone 49.2 4 (3.9) NA NA TT?pCND 80.2 4 (3.9) 0 4 TT alone 67.4 7 (1.5) 2 4 pCND prophylactic central neck dissection, TT total thyroidectomy, LRR locoregional recurrence, CNR central neck recurrence, LNR lateral neck recurrence, NA not available a Medians were provided b Studies that did not mention site of locoregional recurrence, so the number of patients for recurrence site was not available The results of the meta-analysis showed that patients who underwent TT?pCND have a significantly lower incidence of LRR than those underwent TT, which was similar to a previous meta-analysis in 2013.19 We evaluated the central and lateral sites of lymph node recurrences, and PTC recurrence in the central site was lower than that in the lateral region (2.1% vs. 3.1%, respectively). Contrary to the similar recurrence rate at the lateral site, the recurrence at the central site was significantly lower in the TT?pCND group than that in the lateral group (RR = 0.35; P = 0.002), which was not reported in previous meta-analyses.13,18,19 With regards to the extended approach, patients in the TT?pCND group tended to present with recurrence in the lateral region than central region (3.3% vs. 1.1%, respectively); however, patients in the TT group had a similar rate of central and lateral neck recurrence (3.4% vs. 3.3%). As most recurrences in the TT?pCND group occurred in the lateral neck, this indicated that lateral neck recurrence would not have been prevented by CND. pCND for the Locoregional Recurrence of Papillary Thyroid Cancer A 2195 TT=pCND Study or Subgroup Roh, 2007 Choi, 2008 Zuniga, 2009 Perrino, 2009 Costa, 2009 Moo, 2010 Hughes, 2010 Popadich, 2011 So, 2012 Raffaelli, 2012 Lang, 2012 Wang, 2012 Barczynski, 2013 Calo, 2014 Viola, 2015 Lee, 2015 De Carvalho, 2015 Risk Ratio TT Events Total Events Total Weight M-H,Random, 95%CI Year 0.26 [0.01, 4.87] 2007 1.0% 0 3 73 40 48 1.6% 1 2 53 0.55 [0.05, 5.90] 2008 0.70 [0.41, 1.20] 2009 136 19 26 130 16.8% 8.2% 0.39 [0.15, 1.00] 2009 92 5 22 159 8.4% 126 8 9 118 0.83 [0.33, 2.09] 2009 3.5% 45 0.27 [0.06, 1.24] 2010 36 2 6 3.1% 1.67 [0.32, 8.81] 2010 65 78 4 2 0.60 [0.32, 1.13] 2011 259 13 29 347 14.0% 3.0% 119 0.47 [0.09, 2.54] 2012 2 4 113 0.9% 1.51 [0.06, 36.59] 2012 62 124 1 0 1.26 [0.26, 6.06] 2012 82 3 3 103 3.4% 49 0 0 Not estimable 2012 37 0.32 [0.18, 0.57] 2013 358 15 37 282 15.6% 3.0% 65 2 4 220 1.69 [0.32, 9.03] 2014 7 93 0.95 [0.35, 2.59] 2015 7.3% 88 7 153 4 104 4.8% 0.85 [0.23, 3.09] 2015 5 102 4 7 478 2.68 [0.80, 8.98] 2015 5.4% 1969 2468 100.0% Total (95% Cl) Total events 91 165 Heterogeneity: Tau2 = 0.07; Chi2 = 18.53, df = 15 (P =0.24); I2 = 19% Test for overall effect: Z = 2.64 (P = 0.008) B Study or Subgroup Barczynski, 2013 Calo, 2015 Costa, 2009 De Carvalho, 2015 Hughes, 2010 Moo, 2010 Popadich, 2011 Raffaelli, 2012 Roh, 2007 So, 2012 0.66 [0.49, 0.90] 0.01 TT Risk Ratio TT+pCND Events Total Events Total Weight M-H, Random, 95%CI 0.07 [0.02, 0.30] 2 22 282 16.5% 358 0 1 220 65 4.0% 1.12 [0.05, 27.08] 0.94 [0.24, 3.66] 4 4 118 17.9% 126 0.93 [0.04, 19.23] 0 2 478 102 4.5% 2 2 65 10.1% 78 0.83 [0.12, 5.75] 45 0.16 [0.01, 3.25] 0 2 36 4.5% 0.24 [0.08, 0.70] 4 22 347 26.0% 259 1.51 [0.06, 36.59] 1 0 62 124 4.0% 40 0.26 [0.01, 4.87] 0 3 73 4.7% 0.32 [0.03, 3.00] 1 3 113 119 7.7% Total (95% Cl) 1316 1794 100.0% Total events 14 61 Heterogeneity: Tau2 = 0.15; Chi2 = 10.35, df = 9 (P =0.32); I2 = 13% Test for overall effect: Z = 3.12 (P = 0.002) C Study or Subgroup Barczynski, 2013 Calo, 2015 Choi, 2008 Costa, 2009 De Carvalho, 2015 Hughes, 2010 Lang, 2012 Moo, 2010 Popadich, 2011 Roh, 2007 So, 2012 Risk Ratio M-H, Random, 95%CI TT TT+pCND Events Total Events 2 0 40 2 1 48 5 5 126 4 1 45 0 2 78 259 21 11 1 2 119 3 1 82 358 15 13 4 4 102 2 65 4 Total 73 53 118 36 65 347 113 103 282 478 220 0.1 1 TT=pCND TT 10 100 Risk Ratio M-H, Random, 95%CI 0.35 [0.18, 0.68] 0.01 0.1 1 TT=pCND TT Risk Ratio Weight M-H, Random, 95%CI 0.36 [0.02, 7.34] 2.0% 0.55 [0.05, 5.90] 3.2% 0.94 [0.28, 3.15] 11.2% 0.20 [0.02, 1.71] 3.9% 4.18 [0.20, 85.49] 2.0% 0.70 [0.34, 1.43] 26.4% 1.90 [0.17, 20.66] 3.2% 1.26 [0.26, 6.06] 7.0% 0.68 [0.33, 1.41] 25.6% 4.69 [1.19, 18.43] 9.1% 1.69 [0.32, 9.03] 6.3% 1322 1888 100.0% Total (95% Cl) Total events 44 61 Heterogeneity: Tau2 = 0.05; Chi2 = 11.12, df = 10 (P =0.35); I2 = 10% Test for overall effect: Z = 0.28 (P = 0.78) 10 100 Risk Ratio M-H, Random, 95%CI 0.94 [0.61, 1.45] 0.01 0.1 1 TT=pCND TT 10 100 FIG. 2 Forest plot showing a meta-analysis of locoregional recurrence (LRR) for included studies, a overall LRR; b central compartment recurrence; c lateral compartment recurrence In terms of RAI, the TT?pCND group showed a higher rate of RAI ablation therapy (OR = 1.20, 95% CI 1.04–1.39), likely because of upstaging for the higher incidence of central LNM from pCND. Regarding postoperative complications, patients in the TT?pCND group had a significantly higher rate of hypocalcemia and overall 2196 FIG. 3 Forest plot showing a meta-analysis of hypocalcemia for included studies, a temporary hypocalcemia; b permanent hypocalcemia; c temporary hypocalcemia for 6 months as the dividing value; d permanent hypocalcemia for 6 months as the dividing value W. Zhao et al. A TT+pCND TT Odds Ratio Study or Subgroup Events Total Events Total Weight M-H,Random,95%CI 4.54 [1.63, 12.62] 40 7 73 4.1% Roh, 2007 13 1.57 [0.50, 4.90] 6 53 8 48 3.4% Choi, 2008 1.28 [0.50, 3.31] 4.7% Perrino, 2009 92 11 159 8 4.0% 21 5 65 4.42 [1.56, 12.51] Hughes, 2010 78 7.68 [1.61, 36.52] 45 1.9% 14 Moo, 2010 2 36 2.54 [1.29, 4.99] 25 259 14 347 7.9% Popadich, 2011 1.38 [0.81, 2.36] 49 119 38 113 10.9% So, 2012 53 124 3.46 [1.65, 7.27] 6.9% Raffaelli, 2012 62 11 15 2.34 [0.97, 5.66] 82 Lang, 2012 9 103 5.2% 37 21 6.19 [1.90, 20.17] Wang, 2012 49 3.2% 4 2.90 [1.92, 4.38] 37 282 14.5% Barczynski, 2013 109 358 1.80 [1.16, 2.77] 47 102 De Carvalho, 2015 154 478 13.8% 56 153 2.28 [1.28, 4.08] 9.8% Lee, 2015 21 104 25 65 1.88 [1.04, 3.37] 9.7% Calo, 2014 55 220 1614 2132 100.0% 2.37 [1.89, 2.96] Total (95% Cl) Total events 464 374 2 2 2 Heterogeneity: Tau = 0.05; Chi = 17.82, df = 13 (P =0.16); I = 27% Test for overall effect: Z = 7.54 (P < 0.00001) B 0.01 TT+pCND TT Study or Subgroup Events Total Events 48 1 0 Choi, 2008 6 1 92 Perrino, 2009 1 0 45 Moo, 2010 0 78 Hughes, 2010 2 2 Popadich, 2011 2 259 7 119 2 So, 2012 0 1 124 Raffaelli, 2012 2 Lang, 2012 1 82 0 49 3 Wang, 2012 8 358 2 Barczynski, 2013 11 12 102 De Carvalho, 2015 2 Lee, 2015 5 153 10 65 7 Calo, 2015 7 Viola, 2015 18 0 Odds Ratio Total Weight M-H,Random,95%CI 0.36 [0.01, 9.07] 53 3.2% 0.28 [0.03, 2.36] 6.4% 159 0.26 [0.01, 6.58] 36 3.2% 4.28 [0.20, 90.78] 65 3.5% 1.34 [0.19, 9.59] 7.3% 347 3.47 [0.71, 17.07] 9.8% 113 1.52 [0.06, 37.81] 62 3.2% 2.55 [0.23, 28.63] 103 5.3% 0.10 [0.00, 1.99] 37 3.7% 3.20 [0.67, 15.19] 282 10.1% 5.66 [2.42, 13.23] 478 18.7% 1.72 [0.33, 9.05] 104 9.3% 2.53 [0.92, 6.95] 220 16.3% Not estimable 0 Total (95% Cl) 2059 1571 100.0% 13 21 14 25 21 15 49 47 25 40 78 45 259 49 82 119 102 65 7 5 2 14 4 9 38 154 55 73 65 36 347 37 103 113 478 220 7.7% 7.5% 3.9% 13.3% 6.1% 9.5% 16.8% 19.8% 15.4% 0.01 4.54 [1.63, 12.62] 4.42 [1.56, 12.51] 7.68 [1.61, 36.52] 2.54 [1.29, 4.99] 6.19 [1.90, 20.17] 2.34 [0.97, 5.66] 1.38 [0.81, 2.36] 1.80 [1.16, 2.77] 1.88 [1.04, 3.37] TT+pCND TT Odds Ratio Study or Subgroup Events Total Events Total Weight M-H,Random,95%CI 4.28 [0.20, 90.78] Hughes, 2010 65 5.4% 2 0 78 Moo, 2010 0 36 4.9% 0.26 [0.01, 6.58] 1 45 2 259 Popadich, 2011 1.34 [0.19, 9.59] 2 347 10.9% 3 49 0 37 Wang, 2012 0.10 [0.00, 1.99] 5.6% 2 8.0% 82 Lang, 2012 1 103 2.55 [0.23, 28.63] 3.47 [0.71, 17.07] 7 119 So, 2012 2 113 14.6% 5.66 [2.42, 13.23] De Carvalho, 2015 12 102 11 478 27.0% 18 Viola, 2015 Not estimable 7 0 0 7 65 10 220 23.7% Calo, 2015 2.53 [0.92, 6.95] Total (95% Cl) 799 1399 100.0% 2.36[1.10, 5.06] Total events 50 37 2 2 2 Heterogeneity: Tau = 0.37; Chi = 10.71, df = 7 (P =0.15); I = 35% Test for overall effect: Z = 2.22 (P = 0.03) 0.1 1 10 TT+pCND TT 100 Odds Ratio M-H, Random,95%CI 2007 2010 2010 2011 2012 2012 2012 2015 2015 839 1472 100.0% 2.44[1.75, 3.38] Total (95% Cl) Total events 230 288 Heterogeneity: Tau2 = 0.09; Chi2 = 13.35, df = 8 (P =0.10); I2 = 40% Test for overall effect: Z = 5.32 (P < 0.00001) D 100 1.93 [1.05, 3.57] TT+pCND TT Odds Ratio Study or Subgroup Events Total Events Total Weight M-H,Random,95%CI Year Roh, 2007 Hughes, 2010 Moo, 2010 Popadich, 2011 Wang, 2012 Lang, 2012 So, 2012 De Carvalho, 2015 Calo, 2015 0.1 1 10 TT+pCND TT Odds Ratio M-H, Random,95%CI Year 2008 2009 2010 2010 2011 2012 2012 2012 2012 2013 2015 2015 2015 2015 Total events 65 48 Heterogeneity: Tau2 = 0.33; Chi2 = 17.07, df = 12 (P =0.15); I2 = 30% Test for overall effect: Z = 2.11 (P = 0.03) C Odds Ratio M-H, Random,95%CI Year 2007 2008 2009 2010 2010 2011 2012 2012 2012 2012 2013 2015 2015 2015 0.01 0.1 1 10 TT+pCND TT 100 Odds Ratio M-H, Random,95%CI Year 2010 2010 2011 2012 2012 2012 2015 2015 2015 0.01 0.1 1 10 TT+pCND TT 100 pCND for the Locoregional Recurrence of Papillary Thyroid Cancer 2197 morbidity than those in the TT group, which was similar to previous meta-analyses.5,13,18,19 There were no differences in the rate of RLN, wound infection or hematoma formation between the two groups. However, the overall morbidity rate (excluding hypocalcemia) was similar, indicating that complications associated with pCND were actually related to hypocalcemia rather than other surgically related complications. We acknowledge that our meta-analysis has several limitations. First, regarding RAI therapy, only 7 of 17 studies mentioned the RAI therapeutic dose, which can influence the LRR.7,9,14,15,25,29,30 Additionally, the RAI strategies based on the indications for RAI therapy were different among these studies, which can limit the ability to analyze the mechanism of recurrence. Second, the extent of pCND differed among the studies. Unilateral or bilateral pCND may have different effect on outcomes. Third, the follow-up period was relative short, and only 5 of 17 studies reported an overall mean follow-up period of more than 5 years. Therefore, an extended follow-up period would be necessary to detect LRR and distinguish real recurrence and persistent disease. Fourth, the surveillance strategy was not standardized among these studies. Although 14 studies used a combination of serum Tg and neck ultrasonography during the follow-up visit as the fundamental physical examination, the frequency and duration of these visits differed greatly.7–9,12–15,24,26–28,30–33 Additionally, two studies did not mention the clinical examination approaches, and one study used serum Tg alone.11,25,29 Furthermore, the definitions of recurrence were not routinely defined in the studies. Those differences in surveillance also influenced the definition of recurrence and the monitoring of precise time of recurrence. Finally, because of high percentage of the studies were of retrospective design (14/17) with existing selection bias, the quality of included studies was rated as low. Baseline characteristics, such as age, tumor size, extrathyroidal extension, and multifocality, showed significant differences between both groups; thus, any of these risk factors could potentially influence the incidence of recurrence. Moreover, regarding the selection criteria for pCND, 9 of 17 studies were based on the surgeon’s preference, whereas the remaining 8 studies did not mention their methods.8,11,14,15,22–24,30,31 Therefore, we should be cautious with our results regarding these limitations of the previously published data. In conclusion, this meta-analysis showed that pCND significantly reduced the LRR for patients with PTC, specifically in the central region. However, there are some important limitations to pCND, including a higher rate of postoperative RAI ablation and hypocalcemia. Furthermore, the reduction in LRR may be partially related to the increased rate of RAI. We suggest that pCND should not be routinely recommended for treating patients with PTC, even by experienced surgeons. However, more evidence from prospective, multicenter, randomized, controlled trials is needed to address further the true role of pCND in PTC patients, not only for the actual LRR benefits but also for safety reasons. ACKNOWLEDGEMENT This work is supported by the grants from the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-3-005). The authors thank Dr. Lin Ma (Department of General Surgery, Peking Union Medical College Hospital) for furnishing data. DISCLOSURE interest. The authors declare no conflict of any commercial REFERENCES 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. 2. Carty SE, Cooper DS, Doherty GM, Duh QY, Kloos RT, Mandel SJ, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19:1153–8. 3. Pereira JA, Jimeno J, Miquel J, Iglesias M, Munné A, Sancho JJ, et al. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery. 2005;138:1095–101. 4. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. 5. Zhu W, Zhong M, Ai Z. Systematic evaluation of prophylactic neck dissection for the treatment of papillary thyroid carcinoma. Jpn J Clin Oncol. 2013;43:883–8. 6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. 7. Barczynski M, Konturek A, Stopa M, Nowak W. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg. 2013;100:410–8. 8. Lang BHH, Wong KP, Wan KY, Lo CY. Impact of routine unilateral central neck dissection on preablative and postablative stimulated thyroglobulin levels after total thyroidectomy in papillary thyroid carcinoma. Ann Surg Oncol. 2012;19:60–7. 9. So YK, Seo MY, Son YI. Prophylactic central lymph node dissection for clinically node-negative papillary thyroid microcarcinoma: influence on serum thyroglobulin level, recurrence rate, and postoperative complications. Surgery. 2012;151:192–8. 10. Ywata de Carvalho A, Chulam TC, Kowalski LP. Long-term results of observation vs prophylactic selective level VI neck dissection for papillary thyroid carcinoma at a cancer center. JAMA Otolaryngol Head Neck Surg. 2015;141:599–606. 11. Choi SJ, Kim TY, Lee JC, Shong YK, Cho KJ, Ryu JS, et al. Is routine central neck dissection necessary for the treatment of papillary thyroid microcarcinoma? Clin Exp Otorhinolaryngol. 2008;1:41–5. 12. Perrino M, Vannucchi G, Vicentini L, Cantoni G, Dazzi D, Colombo C, et al. Outcome predictors and impact of central node 2198 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. dissection and radiometabolic treatments in papillary thyroid cancers B 2 cm. Endocr Relat Cancer. 2009;16:201–10. Zetoune T, Keutgen X, Buitrago D, Aldailami H, Shao H, Mazumdar M, et al. Prophylactic central neck dissection and local recurrence in papillary thyroid cancer: a meta-analysis. Ann Surg Oncol. 2010;17:3287–93. De Carvalho AY, Chulam TC, Kowalski LP. Long-term results of observation vs prophylactic selective level VI neck dissection for papillary thyroid carcinoma at a cancer center. JAMA Otolaryngol Head Neck Surg. 2015;141:599–606. Calò PG, Pisano G, Medas F, Marcialis J, Gordini L, Erdas E, et al. Total thyroidectomy without prophylactic central neck dissection in clinically node-negative papillary thyroid cancer: is it an adequate treatment? World J Surg Oncol. 2014;12:152. Carling T, Carty SE, Ciarleglio MM, Cooper DS, Doherty GM, Kim LT, et al. American Thyroid Association design and feasibility of a prospective randomized controlled trial of prophylactic central lymph node dissection for papillary thyroid carcinoma. Thyroid. 2012;22:237–44. Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope. 2009;119:1135–9. Shan CX, Zhang W, Jiang DZ, Zheng XM, Liu S, Qiu M. Routine central neck dissection in differentiated thyroid carcinoma: a systematic review and meta-analysis. Laryngoscope. 2012;122:797–804. Lang BH, Ng SH, Lau LL, Cowling BJ, Wong KP, Wan KY. A systematic review and meta-analysis of prophylactic central neck dissection on short-term locoregional recurrence in papillary thyroid carcinoma after total thyroidectomy. Thyroid. 2013;23:1087–98. Wang TS, Cheung K, Farrokhyar F, Roman SA, Sosa JA. A metaanalysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann Surg Oncol. 2013;20:3477–83. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. Wang TS, Evans DB, Fareau GG, Carroll T, Yen TW. Effect of prophylactic central compartment neck dissection on serum thyroglobulin and recommendations for adjuvant radioactive iodine in patients with differentiated thyroid cancer. Ann Surg Oncol. 2012;19:4217–22. Zuniga S, Sanabria A. Prophylactic central neck dissection in stage N0 papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135:1087–91. W. Zhao et al. 24. Raffaelli M, De Crea C, Sessa L, Giustacchini P, Revelli L, Bellantone C, et al. Prospective evaluation of total thyroidectomy versus ipsilateral versus bilateral central neck dissection in patients with clinically node-negative papillary thyroid carcinoma. Surgery (United States). 2012;152:957–64. 25. Viola D, Materazzi G, Valerio L, Molinaro E, Agate L, Faviana P, et al. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab. 2015;100:1316–24. 26. Lee DY, Oh KH, Cho JG, Kwon SY, Woo JS, Baek SK, et al. The benefits and risks of prophylactic central neck dissection for papillary thyroid carcinoma: prospective cohort study. Int J Endocrinol. 2015. doi:10.1155/2015/571480. 27. Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007;245:604–10. 28. Costa S, Giugliano G, Santoro L, Ywata De Carvalho A, Massaro MA, Gibelli B, et al. Role of prophylactic central neck dissection in cN0 papillary thyroid cancer. Acta Otorhinolaryngol Ital. 2009;29:61–9. 29. Moo TA, McGill J, Allendorf J, Lee J, Fahey T 3rd, Zarnegar R. Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg. 2010;34:1187–91. 30. Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148:1100–6; discussion 1106–7. 31. Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery. 2011;150:1048–57. 32. White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World J Surg. 2007;31:895–904. 33. Shen WT, Ogawa L, Ruan D, Suh I, Duh QY, Clark OH. Central neck lymph node dissection for papillary thyroid cancer: the reliability of surgeon judgment in predicting which patients will benefit. Surgery. 2010;148:398–403. 34. Carling T, Long WD 3rd, Udelsman R. Controversy surrounding the role for routine central lymph node dissection for differentiated thyroid cancer. Curr Opin Oncol. 2010;22:30–4.