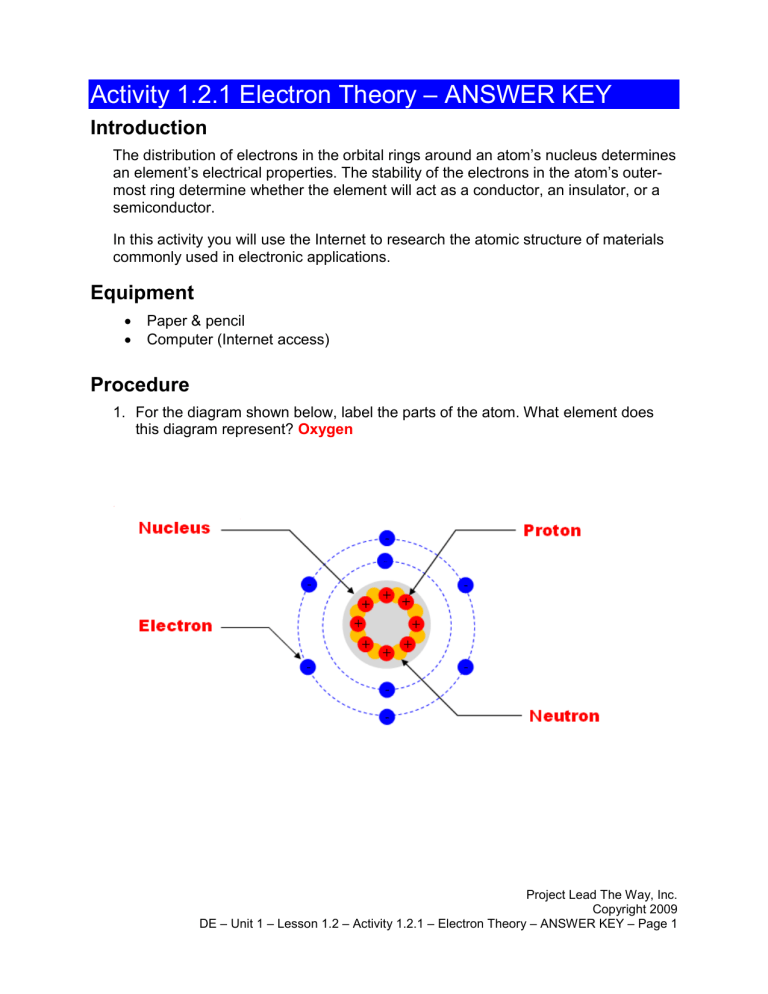
Activity 1.2.1 Electron Theory – ANSWER KEY Introduction The distribution of electrons in the orbital rings around an atom’s nucleus determines an element’s electrical properties. The stability of the electrons in the atom’s outermost ring determine whether the element will act as a conductor, an insulator, or a semiconductor. In this activity you will use the Internet to research the atomic structure of materials commonly used in electronic applications. Equipment Paper & pencil Computer (Internet access) Procedure 1. For the diagram shown below, label the parts of the atom. What element does this diagram represent? Oxygen Project Lead The Way, Inc. Copyright 2009 DE – Unit 1 – Lesson 1.2 – Activity 1.2.1 – Electron Theory – ANSWER KEY – Page 1 2. Shown below are the electron orbital rings for the elements Lead (Pd) and Tin (Sn). Complete the diagrams by labeling the number of electrons that belong in each of the orbital rings. Based on their electron configuration, would tin and lead be good conductors? Yes Do you remember what is made from tin and lead? Solder Conclusions 1. What are the three basic particles that make up all atoms? 2. What is the primary characteristic of an element that makes it a good conductor? 3. Give three examples of materials that make good conductors. 4. What is the primary characteristic of an element that makes it a good insulator? 5. Give three examples of materials that make good insulators. Project Lead The Way, Inc. Copyright 2009 DE – Unit 1 – Lesson 1.2 – Activity 1.2.1 – Electron Theory – ANSWER KEY – Page 2


