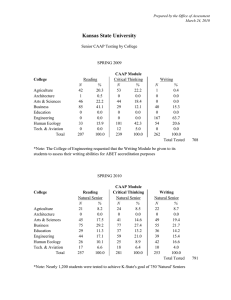
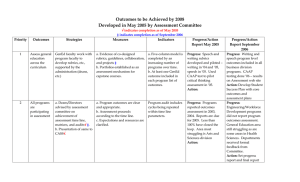
Name:___________________________________ Period:_____________ Date:_________________ Practice Problems Stoichiometry Mass-Mass Conversions #1. First balance the equation then determine how much acetylene gas (C2H2) is produced by adding water to calcium carbide (CaC2). CaC2 (s) + H2O(l) C2H2(g) + Ca(OH)2 (aq) How many grams of acetylene are produced by adding water to 5.00 g of CaC2? Knowns: mass of CaC2 mole ratio of CaC2/ C2H2 molar mass of C2H2 molar mass of CaC2 #2. First balance the equation then figure out how many grams of dinitrogen tetrafluoride can be produced from 225 g of fluorine? F2(g) + Knowns: NH3(g) N2F4(g) + mass of F2 mole ratio of N2F2/F2 molar mass of N2F2 molar mass of F2 HF(g)