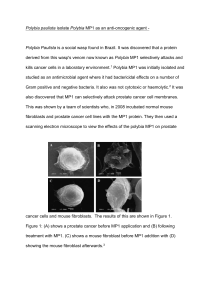
Thomas Parsley 77549 Word count 905 Polybia paulista isolate Polybia MP1 as an anti-oncogenic agent - Gavin Knight Polybia Paulista is a social wasp found in Brazil. It was discovered that a protein derived from this wasp's venom now known as Polybia MP1 selectively attacks and kills cancer cells in a laboratory environment.1 Polybia MP1 was initially isolated and studied as an antimicrobial agent where it had bactericidal effects on a number of Gram positive and negative bacteria. It also was not cytotoxic or haemolytic.2 It was also discovered that MP1 can selectively attack prostate cancer cell membranes. This was shown by a team of scientists who, in 2008 incubated normal mouse fibroblasts and prostate cancer cell lines with the MP1 protein. They then used a scanning electron microscope to view the effects of the polybia MP1 on prostate cancer cells and mouse fibroblasts. The results of this are shown in Figure 1. Figure 1: (A) shows a prostate cancer before MP1 application and (B) following treatment with MP1. (C) shows a mouse fibroblast before MP1 addition with (D) showing the mouse fibroblast afterwards.3 Thomas Parsley 77549 Word count 905 The figure shows that there is significant damage to the membrane of the prostate cancer cell with no effect on the fibroblast. The scientists also determined that bladder cancer cell lines were also killed by polybia MP1.3 Originally the mechanism for this selectivity and for the disruption of the cell membrane was unclear. Now it is believed that two phospholipids known as phosphatidylethanolamine and phosphatidylserine are what allows MP1 to selectively attack the membranes of cancer cells.4 It has been shown previously that phosphatidylserine, which is normally distributed on the inside leaflet of the cell membrane, is found on the outside of cancer cells and other pathological cells (such as apoptotic cells).5 This provides evidence for why cancer cells are targeted by MP1 as the cancer cells are the only cells that have phosphatidylserine and phosphatidylethanolamine accessible to the protein. To test the hypothesis the scientists created giant unilamellar vesicles (GUVs) with varying amounts of phosphatidylethanolamine and phosphatidylserine and then exposed them to MP1. They then exposed these model membranes to 3 fluorescent dyes of varying molecular mass Then using fluorescence microscopy they were able to view the GUVs they had created and see which dyes had entered the GUVs thus allowing them to determine the size of the pores created. They then used multiple other processes to corroborate the results from the first experiment. This resulted in the discovery that phosphatidylserine significantly increases the bound concentration of lipid to MP1 (by a factor of 7-8) while phosphatidylethanolamine increased the permeability of the membrane by allowing MP1 to create larger pores.5 However it must be noted that just because MP1 selectively attacks cancer cells in a laboratory environment it does not mean that MP1 is a clinically viable cancer Thomas Parsley 77549 Word count 905 treatment. To be clinically viable the protein would need to be proved safe for use in the human body and more studies would be needed to reach this conclusion. In addition the breakdown of the cell membrane causes cell death through important molecules exiting the cell. These molecules include RNA and different varieties of proteins.6 This is a mode of cell death can be described as necrosis and any material within the cell could well diffuse out of it. This would include any substances that may be harmful when outside of the cells such as lysosomes. In addition to this the presence of these dead necrotic cells would induce an inflammatory response from the body’s immune system which could cause further complications.7 One of the professors instead suggests that the two phospholipids targeted by MP1 could provide new target for combination therapies where multiple drugs are used in combination to help attack the tumour. This would be useful as any drug developed that targets this molecule would be a new class of anticancer drug. 6 Combination therapies were developed with the idea that using multiple drugs with multiple mechanisms of action would be a very efficient way of treating cancer. 8 Due to this having a whole new mode of action and being a whole new class of drugs this could be very useful for using with other existing drugs in combination chemotherapy. Polybia MP1 has also not been tested with all the cell types in the human body. Since human somatic cells have a huge variety of different cell surface markers involved in many different processes it cannot be proved that MP1 will not react with any of these other surface markers without extensive testing.9 If MP1 were to react with other molecules this could mean it is not clinically viable as it could pose a severe risk to the patient's health. Need to consider exploring this further. What Thomas Parsley 77549 Word count 905 about differential sensitivities of cancer cells? Do all cancer cells repond in the same way? In conclusion the MP1 protein is clearly an anti-oncogenic agent because it attacks and kills cancer cells and has been shown to inhibit the growth of a variety of different cancers. However this does not necessarily mean it can be used as a treatment for cancer in humans due to a variety of different reasons outlined in this report. To determine whether it would be an effective cancer treatment extensive testing of the drug would need to be carried out both on cell cultures and through other methods. The MP1 protein’s specificity to the phospholipids phosphatidylethanolamine and phosphatidylserine presents an opportunity for the creation of new drugs that target these molecules that can be found on the surface of cancer cells. This could lead to a variety of new treatments derived from the protein MP1. So, to summarise, Polybia MP1 is an anti-onconogenic agent in the sense that it kills cancer cells however it may not be suitable for treatment of cancer within human patients. References: 1. Sciencedaily. Sciencedaily.com. [Online]. Available from: http://www.sciencedaily.com/releases/2015/09/150901134941.htm [Accessed 1 December 2015]. 2. De souza, B.M, Da silva, A.V, Arcuri, H.A, Palma, M.S, Neto, J.R. et al Characterization of two novel polyfunctional mastoparan peptides from the venom of the social wasp Polybia paulista. Peptides. 2009;30(8): 1387–1395. Thomas Parsley 77549 Word count 905 3. Wang K, Zhang B, Zhang W, Yan J, Li J, Wang R et al. Antitumor effects, cell selectivity and structure–activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides. 2008;29(6):963-968. 4. Leite N, Aufderhorst-Roberts A, Palma M, Connell S, Neto J, Beales P et al. PE and PS Lipids Synergistically Enhance Membrane Poration by a Peptide with Anticancer Properties. Biophysical Journal. 2015;109(5):936-947. 5. 3. Zwaal R, Comfurius P, Bevers E. Surface exposure of phosphatidylserine in pathological cells. CMLS, Cell Mol Life Sci. 2005;62(9):971-988. 6. Phys.org. Brazilian wasp venom kills cancer cells by opening them up [Internet]. 2015 [cited 3 December 2015]. Available from: http://phys.org/news/2015-09-brazilian-wasp-venom-cancer-cells.html 7. Cabana E. Modes of Cell Death [Internet]. mozcom. 2015 [cited 3 December 2015]. Available from: http://www2.mozcom.com/~emcdvm/path02.html 8. Chabner B, Thompson E. Combination Cancer Therapy - Cancer [Internet]. MSD Manual. 2015 [cited 3 December 2015]. Available from: http://www.msdmanuals.com/home/cancer/prevention-and-treatment-ofcancer/combination-cancer-therapy 9. Cooper G. The Cell: A Molecular approach. 2nd ed. Sunderland (MA): Sinauer Associates; 2015. Available from:http://www.ncbi.nlm.nih.gov/books/NBK9898/