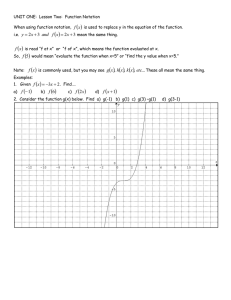
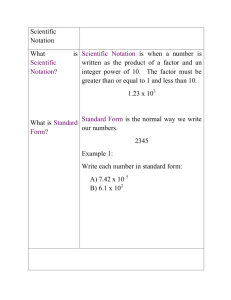
Name:_____________________________ Date:______________ Class Period:_________ Unit One Review 1. What is the main difference between chemical and physical change? Give an example of each. 2. Classify each of the following as either a physical or chemical change. a. Tearing a sheet of paper b. Melting a piece of wax c. Burning a log 3. How do you decide whether a sample is a solid, liquid, or gas? 4. Contrast mixtures with pure substances. 5. How can you tell the difference between an element, compound, and mixture? 6. What is the density of a block of marble that occupies 3110. cm3 and has a mass of 853 g? (Remember sig figs!) 7. Write conversion factors for each of the following. a. 10.5 g (------------) = ? kg b. 1.2 L (---------) = ? mL 8. What is the percentage error for a mass measurement of 17.7 g, given that the correct value is 21.2g? 9. How many significant figures are in each of the following measurements? a. 804.05 g b. 0.0144030 km c. 1002 m d. 400 mL e. 30000. Cm f. 0.000625000 kg 10. Carry out the following calculations. Express each answer to the correct number of significant figures. a. 5.44 m – 2.6103 m b. 2.4 mL x 15.82 mL c. The area of rectangular crystal surface that measure 1.34 cm by 0.7488 cm. (A=l x w) d. The sum of 2.099g and 0.05681 g 11. Write the following numbers in scientific notation. a. 560,000 b. 33,400 c. 0.0004120 12. Perform the following operations. Express each answer in scientific notation. a. (1.54 x 10-2) + (2.86 x 10-1) b. (7.023 x 109) – (6.62 x 107) c. (8.99 x 10-4) x (3.57 x 104) d. (2.17 x 10-3) / (5.022 x 104) 13. What is the volume, in cubic meters, of a rectangular solid that is 0.25 m long, 6.1 m wide, and 4.9 m high? 14. Round each of the following measurements to the number of significant figures indicated. a. 67.029 to three significant figures b. 0.15 L to one significant figure c. 52.8005 mg to five significant figures d. 3.17497 mol to three significant figures 15. What should you do if you find a crack in your beaker during an experiment? 16. What is the difference between accuracy and precision? 17. What units are used to measure volume? Mass? 18. Identify the name given to each group number on the periodic table: a. b. c. d. e. Group 1 Group 2 Groups 3-12 Group 17 Group 18