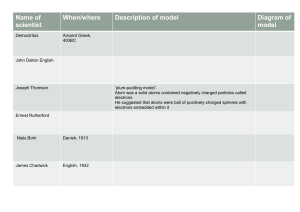
Atomic Structure History of Atomic Theory Compiled & created by Ms Shivani Kaur Sihra Democritus (460 - 370 BC) Was the first person to come up with the idea of atom Believed that all matter was composed of indivisible particles he called “ATOMS” Which is derived from the Greek word “Atomos” – meaning indivisible He also believed that different atoms: Are different sizes Have different properties Other philosophers of that time did not agree with his theories. John Dalton (1766-1844) • Dalton is the “Father of Atomic Theory” • Dalton’s ideas were so brilliant that they have remained essentially intact up to the present time and has only been slightly corrected. Dalton’s Atomic Theory (1803) aka: 5 Postulates 1. All matter is composed of extremely small particles called atoms. (I agree with Democritus!) 2. All atoms of a given element are identical, having the same: - size - mass - chemical properties. 3. All atoms of different elements are different. Dalton’s Atomic Theory (1803) aka: 5 Postulates 4. Atoms cannot be created, divided into smaller particles, or destroyed. **In a chemical reaction, atoms of different elements are separated, joined or rearranged. They are never changed into the atoms of another element. We will learn more later** 5. Atoms combine in definite whole number ratios to make compounds (you can’t have a ½ of a Carbon bonding with Oxygen; it’s a whole atom or no atom) Dalton’s Atomic Model •Based on Dalton’s Atomic Theory (5 postulates), most scientists in the 1800s believed that the atom was like a tiny solid ball that could not be broken up into parts. •Dalton was credited for the three Atomic Laws that were proven after his time. Dalton’s Atomic Laws 1. Law of Conservation of Mass Matter cannot be created or destroyed in any physical or chemical process, just transferred. 2. Law of Constant Composition When atoms combine to form molecules, the ratio of atoms is constant. Example – H2O will always have 2 times as many Hydrogen atoms as Oxygen. Dalton’s Atomic Laws 3. Law of Multiple Proportions – if two elements can combine to form more than one compound, then the ratio of the second element combined with a certain mass of the first element is always a ratio of small whole numbers. Example: CO vs. CO2 Formula Ratio of N:O The Law of Conservation of Mass •When a chemical reaction occurs, mass is neither created nor destroyed but only changed. JJ Thomson (1856-1940) • Used cathode rays to prove that Dalton’s Solidball model could be broken into smaller particles •Thomson is credited with discovering electrons Cathode Ray Tubes • Cathode rays had been used for some time before Thompson’s experiments. A cathode ray tube is a tube that has a piece of metal, called an electrode, at each end. Each electrode is connected to a power source (battery). • When the power is turned on, the electrodes become charged and produce a glow in the tube which travels from cathode, across the tube to the anode. A Maltese cross made of thin mica is placed in the between the cathode and the walls of the tube. When the experiment is repeated the glow doe not extend to the other end. The shadow of the cross is produced behind it making Thomson believe that the glow was a beam of light travelling like a wave in a straight line . ----------------------------++++++++++++++ • Thomson put the tube in a magnetic field. He predicted that the stream would travel in a straight path. • Instead, he found that the path curved away from a negatively charged plate and toward a positively charged plate Why? •Like charges repel each other, and objects with unlike charges attract each other, Thomson concluded that the glow had a stream of negatively charged particles which he named electrons . The particulate nature is evident from a modification of the same experiment where a paddle wheel is placed in the path of the rays and it starts spinning as if it has been hit by fast moving particulate bodies. •Thompson Concluded: •Cathode rays are made up of invisible, negatively charged particles called Electrons. •These electrons had to come from the matter (atoms) of the negative electrode. •Since the electrodes could be made from a variety of metals, then all atoms must contain electrons! Thomson’s Plum Pudding Model •Thomson’s Plum Pudding model is a + charge sphere that has (- )charged electrons scattered inside, like “raisins” in “plum pudding”. •Overall, the atom is neutral atom because the atom had the same number of positive and negative charges. •From Thomson’s experiments, scientists concluded that atoms were not just neutral spheres, but somehow were composed of electrically charged particles. •The balance of positive and negative charge supports the neutral atom. Rutherford (1871-1937) Took Thomson’s Plum Pudding Model and added to it Used the “Gold Foil Experiment” to discover the existence of: An atomic Nucleus Protons (in later experiments) You must be able to explain the Gold Foil Experiment…it will be on the CST Gold Foil Experiment Rutherford directed a narrow beam of alpha particles (+ charges) at a thin piece of gold foil. Based on observations from other experiments involving alpha particles, he predicted that the (+) charges would go through the foil The Gold Foil Experiment Results from Gold Foil Experiment •Rutherford found that every once and a while, a + particle was deflected bounced back. (about 1% of the time) •Why? •Because the + charge hit a central mass of positive charge and was repelled. Conclusions from Rutherford’s Gold Foil Experiment (memorize this!) THE NUCLEAR MODEL ATOM • The atom contains a positively charged “nucleus” •This nucleus contains almost all of the mass of the atom, but occupies a very small volume of the atom. •The negatively charged electrons occupied most of the volume of the atom. • The atom is mostly empty space. Rutherford’s Planetary Model • To explain his observations, Rutherford developed a new model •The electrons orbit the nucleus like the planets revolve around the sun. Bohr (1885-1962) Worked in Rutherford’s lab Wondered why – electrons are not attracted to the + nucleus and cluster around it Disproved Rutherford’s Planetary Model Experimented with light and its interaction with matter to develop a new model. Bohr’s Energy Level Model Energy Level Model: Electrons are arranged in circles around the nucleus. Each circle has a different energy. •Electrons are in constant motion, traveling around the circle at the speed of light. •Electrons can “jump” from one circle to the next •But they can’t go to the nucleus they traveling too fast to be fully attracted. Bohr’s Energy Level Model He proposed the following: 1. Protons and neutrons are in the nucleus 2. Electrons can only be certain distances from the nucleus. 3. The electrons orbit the nucleus at fixed energy levels. 4. The electrons must absorb or emit a fixed amount of energy to travel between these energy levels Review Who is the father of atomic theory? Dalton What was the first model of the atom? Dalton’s Tiny Ball Model What are Dalton’s 3 Laws? Law of Conservation of Mass, Law of Constant Composition, Law of Multiple Porportion Review How were Thomson’s and Dalton’s model different? Dalton’s model was 1 sphere that cannot be divided, Thomson had the plum pudding where electrons are randomly spread throughout a positively charged sphere. What did Thomson find out? Atoms have electrons, they have a - charge Review What were Rutherford’s conclusions from the Gold Foil Experiment? Atom has a positively charged nucleus electrons are outside, atoms are mostly empty Nucleus contains most of the mass.