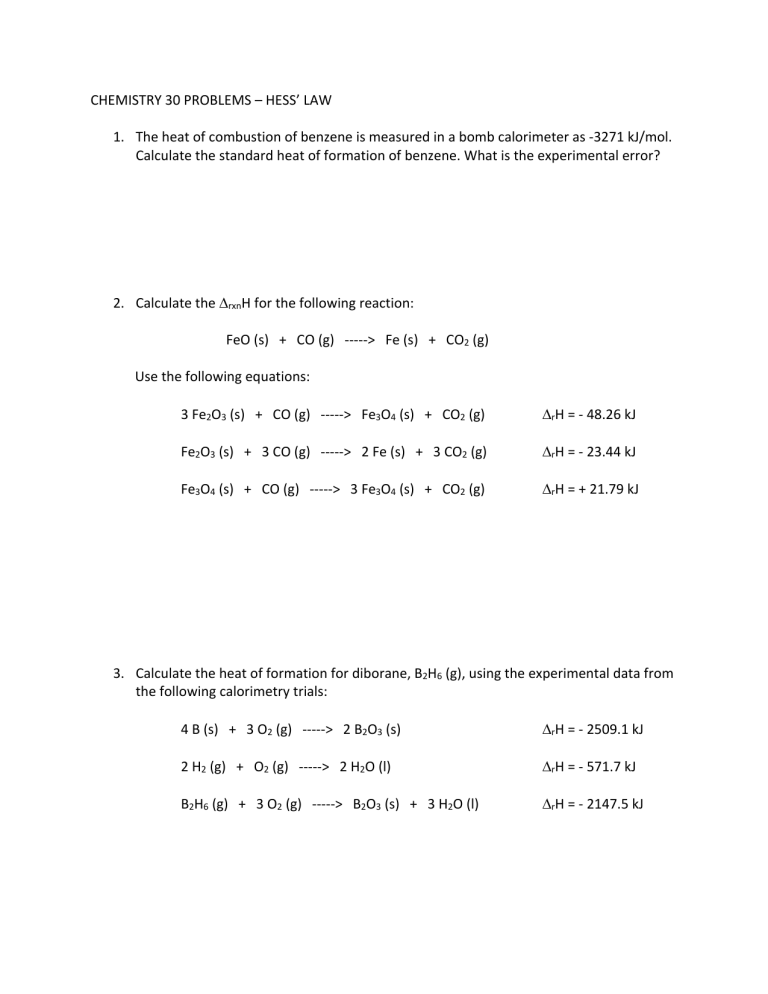
CHEMISTRY 30 PROBLEMS – HESS’ LAW 1. The heat of combustion of benzene is measured in a bomb calorimeter as -3271 kJ/mol. Calculate the standard heat of formation of benzene. What is the experimental error? 2. Calculate the DrxnH for the following reaction: FeO (s) + CO (g) -----> Fe (s) + CO2 (g) Use the following equations: 3 Fe2O3 (s) + CO (g) -----> Fe3O4 (s) + CO2 (g) DrH = - 48.26 kJ Fe2O3 (s) + 3 CO (g) -----> 2 Fe (s) + 3 CO2 (g) DrH = - 23.44 kJ Fe3O4 (s) + CO (g) -----> 3 Fe3O4 (s) + CO2 (g) DrH = + 21.79 kJ 3. Calculate the heat of formation for diborane, B2H6 (g), using the experimental data from the following calorimetry trials: 4 B (s) + 3 O2 (g) -----> 2 B2O3 (s) DrH = - 2509.1 kJ 2 H2 (g) + O2 (g) -----> 2 H2O (l) DrH = - 571.7 kJ B2H6 (g) + 3 O2 (g) -----> B2O3 (s) + 3 H2O (l) DrH = - 2147.5 kJ





