SEMINAR
Presented by
DR. Tareq Rahman
DR.Md.Sazzadul Alam
Resident , phase A,
Neonatology,
BSMMU.
ACID-BASE DISORDERS
Terms
Acid
Any substance that can yield a hydrogen ion
(H+) when dissolved in water
Release of proton or H+
Base
Substance that can yield hydroxyl ions (OH-)
Accept protons or H+
pK
Negative log of the ionization constant of an
acid
Strong acids would have a pK <3
Strong base would have a pK >9
pH
Negative log of the hydrogen ion concentration
pH= pK + log([base]/[acid])
Represents the hydrogen concentration
Base Excess (BE) :
It refers to the change in the concentration of buffer
base ( BB ) from it’s normal value . Base excess refers
principally to the [ HCO3- ]
BE are only influenced only by metabolic process.
Normal range : -2 to + 2 mEq/L in arterial blood.
Increase HCO3- : positive base excess ( metabolic
alkalosis)
Decrease HCO3- : negative base excess ( metabolic
acidosis )
Acidemia
pH less than 7.35
Alkalemia
pH greater than 7.45
Normal pH is 7.35-7.45
HCO₃: 20-28 mEq/L
Pco₂: 35-45 mm Hg
Acidosis: pathological process that causes an
increase in hydrogen ion concentration
Alkalosis: pathological process that causes a
decrease in hydrogen ion concentration
Compensation
If underlying problem is metabolic,
hyperventilation or hypoventilation can help :
respiratory compensation.
If problem is respiratory, renal mechanisms can
bring about metabolic compensation.
Buffer
Combination of a weak acid and /or a weak base
and its salt
What does it do?
Resists changes in pH
The
pH at which a buffer is 50% dissociated is
it’s pK. The best physiologic buffers have pK
close to 7.4.
Buffer in body fluid
Blood :
Bicarbonate buffer system
plasma protein buffer system
Haemoglobin buffer system
Phosphate buffer system
Interstitial fluid :
Bicarbonate buffer system
Phosphate buffer system
Cont…
Intracellular fluid :
Phosphate buffer system
protein buffer system
CSF :
Phosphate buffer system
Bicarbonate buffer system
Tissue Buffer :
Kidney :
Bicarbonate buffer
Phosphate buffer
Ammonia buffer
Muscle :
Bicarbonate buffer
Bone :
Hydroxyapatite buffer
Regulation of pH
Direct relation of the production and retention of
acids and bases
Systems
Respiratory Center and Lungs
Kidneys
Buffers
Found in all body fluids
Weak acids good buffers since they can tilt a
reaction in the other direction
Strong acids are poor buffers because they make
the system more acid
Renal Buffering System :
The renal buffer system uses bicarbonate,
phosphate and ammonium buffers. Kidney
maintain acid-base balance in three ways: secrete
H+, reabsorb bicarbonate, or produce new
bicarbonate.
The renal responses to abnormal acid load
are –
increased secretion of H+
increased HCO3- reabsorption and HCO3generation
increased excretion of titrable acid and NH4+ (
mainly )
The renal responses to abnormal alkali load
are –
increased HCO3- excretion in urine
increased excretion of phosphate buffer base
Suppression of ammonia secretion
Reabsorption of bicarbonate
Excretion of titrable acid
Excretion of ammonium
Anion gap
The anion gap is the difference in the measured
cation ( Sodium and potassium) and the measured
anions ( Bicarbonate and chloride) in plasma.
Anion gap= ([Na+] + [K+]) − ([Cl−] - [HCO3−])
Normal value: 8-16 mmol/L
It is also the difference between unmeasured
anions and unmeasured catioions.
Anion gap is increased when there is increase in
unmeasured anions.
Acid- Base disorders
Metabolic acidosis
Metabolic alkalosis
Respiratory acidosis
Respiratory alkalosis
Mixed disorder
Metabolic acidosis
Bicarbonate deficit - blood concentrations of
bicarbonate drop below 20mEq/L
3 basic mechanisms:
1. Loss of bicarbonate from body
2. Impaired ability to excrete acid by kidney
3. Addition of acid to the body
Causes:
Normal anion gap
2. Increased anion gap
1.
Normal anion gap:
Diarrhoea , fistula
Renal tubular acidosis
Urinary tract diversion
Acetazolamide , ammonium chloride
Increased anion gap:
o Lactic acidosis
o Tissue hypoxia:
Shock
Hypoxemia
Severe anaemia
o Liver failure
o Malignancy
o MedicationsMetformin,Propofol
Ketoacidosis: Diabetic KA
Starvation KA
Alcoholic
Kidney failure
Poisoning: Ethylene glycol
Methanol
Salicylate
Paraldehyde
Inborn errors of metabolism
stimulation of
SNS
- tachycardia
- vasoconstriction
- depression of
contractility
- arythmias
(hyperkalemia)
HYPERVENTILATION
“KUSSMAUL RASPIRATION”
Decreased
HCO3
Clinical feature of
metabolic acidosis:
Features of underlying
cause
Headache,lethergy,
Chronic MA- failure to
thrive
Arrythmia
Compensatory
hyperventilation(Kussma
ul respiration)
Coma
Death
Total bicarbonate deficit (mEq)
⁼0.3 ₓ weight(Kg)ₓ( desired- actual serum HCO3-)
investigation :
ABG analysis
ECG
S . Electrolytes
Management of MA
General measures
Put the patient in the resuscitation area, or transfer to a high
dependency area as soon as feasible.
Put the patient on an ECG monitor, SaO2 monitor and BP/HR
monitor.
In patients who are clinically unwell and have deteriorating SaO2
levels or conscious levels, consider intubation and assisted
ventilation, after taking senior A&E/medical/anaesthetic advice.
Get large-bore IV access (a central venous line may be needed)
and rehydrate aggressively.
Use colloids if necessary.
Consider catheterisation to monitor urine output and obtain
urine for analysis. If there is any possibility of drug or toxin
ingestion, give initial therapies such as activated
charcoal/chelating agents/emetics,
Management of MA
In diabetic ketoacidosis: Insulin
In lactic acidosis due to hypovolemia or shock
:Restoration of adequate perfution with I/V
fluid
In salicylate poisoning: alkali administration
increases renal clearance and decrease the
amount of salicylate in brain cells.
Management cont…
Oral base therapy is given to children with chronic
metabolic acidosis.
Citrate solutions are available as sodium citrate,
potassium citrate and a 1:1 mix of sodium citrate
and potassium citrate.
I/V base can be used in acute MA where a rapid
response is necessary. Sodium bicarbonate is given
as 1 mEq/Kg I/V bolus in emergency situation.
Management cont…
Tris-hydroxymethyl aminomethane is an option
for patient with metabolic acidosis and respiratory
acidosis, because it neutralises acid without
releasing CO₂. It difuses into cells and therefore
provides intracellular buffering.
in patient with renal insufficiency:
Haemodialysis
Peritoneal dialysis
Metabolic alkalosis
Metabolic alkalosis is
elevation of arterial pH , an
increase in serum {HCO3-}
and an decrease in serum
PaCO2 as a result of
compensatory
hyperventilation.
.
\
Causes of metabolic alkalosis
Chloride-responsive (Urinary chloride < 15
mEq/L)
Gastric losses:
Emesis
Nasogastric
suction
Congenital hupertrophic pyloric stenosis .
Diuretics (loop or thiazide)
Chloride-losing diarrhea
Chloride-deficient formula
Cystic fibrosis
Post-hypercapnia
Causes of metabolic alkalosis
Chloride resistant (urinary chloride> 20 mEq/L)
High blood pressure:
Adrenal
adenoma or hyperplasia
Glucocorticoid-mediable aldosteronism
Renin-secreting tumor
17β-Hydroxylase deficiency
11β-Hydroxylase deficiency
Cont..
Cushing syndrome
11β-Hydroxysteroid dehydrogenase deficiency
Normal blood pressure:
Gitelman syndrome
Bartter syndrome
Base administration
Pathogenesis of metabolic alkalosis
Loop Diuretic
Vomiting
K+ loss
H+
Shift of
ions into cells
H+ loss
Na+ and Cl- loss
Block Na+
reabsorption
ECF volume
Renin
Angiotensin II
Aldosterone
Reabsorption of Na+ In
eaxchange for H+ ni.e. lossof H+
Plasma HCO3METABOLIC
ALKALOSIS
Na+ delivery
to distal tubule
Clinical manifestation
Depends on underlying disesses
Features of volume depletion
Hypertension
Muscle weakness –hypokalaemia
Muscle cramps , tetany , cardiac arrhythmia hypocalcaemia
Inv and treatment
ABG analysis
ECG
S . Electrolytes
Treatment
Treatment of underlying disorder .
Intervention only moderate (>32 meq/l) to severe m
alkalosis.
Addition of PPI .
RESPIRATORY ACIDOSIS
Caused by hyperkapnia due to ypoventilation
Characterized by a pH decrease and an
increase in CO2
pH
CO2
CO2
CO CO2
2
CO2
CO2
CO2CO2
CO2
CO2
pH
CO2
CO2
CO2
39
HYPOVENTILATION
Elimination of CO2
+
H
pH
40
Causes of respiratory acidosis
CNS depression
Encephalitis
Head trauma
Brain tumor
Stroke
Increased intracranial pressure
Medications:
Narcotics
Barbiturates
Cont..
Guillain-Barré syndrome
Poliomyelitis
Spinal muscular atrophies
Botulism
Myasthenia
Multiple sclerosis
Spinal cord injury
Causes
RESPIRATORY MUSCLE WEAKNESS
Muscular dystrophy
Hypothyroidism
Hypokalemia
Pulmonary diseses
Pneumonia
Pneumothorax
Asthma
Bronchiolitis
UPPER AIRWAY DISEASE
Aspiration
Compensations for Respiratory Acidosis
Acute respiratory acidosis
HCO3 increases by 1 for every 10 mm increases in
pCO2
Chronic respiratory acidosis
HCO3 increases by 3 for every 10 mm increases in
pCO2
Clinical manifestation
Tachypneic
CNS feature headache, cofusion,seizure
CVS feature-cardiac arrhythmia.
Treatment
Adequate ventilation initiated
If Pc02 >75 usually required mechanical ventilation.
Treatment of underlying cause .
RESPIRATORY ALKALOSIS
Cause is Hyperventilation
Leads to eliminating excessive amounts of CO2
Decrease in H+
CO2
CO2
CO2
CO2
CO2
CO2
CO2
CO2
CO2
CO2
CO2
47
HYPERVENTILATION
Elimination of CO2
+
H
pH
48
Causes of respiratory alkalosis
Pneumonia
Cyanotic heart disease
Asthma
Laryngospasm
Aspiration
Pulmonary embolism
causes
• LUNG RECEPTOR STIMULATION
• Pneumonia , pulmnary edema , pulmonary embolism
• Asthma
• CENTRAL STIMULATION
• Central nervous system disease:
• Encephalitis , meningitis
• Anxiety (panic attack)
• Psychogenic hyperventilation or anxiety
• Liver failure
• Sepsis
Clinical feature
Usually asymtomatic
Chest tightness,palpitation , paresthesias
tetany, , seizure , muscle cramps .
Investigation and treatment
ABG analysis
Xray chest
Accorting to cause
Treatment
According to cause
Assurance
Rebreathing into paper bag
Mixed Acid-Base Disorders
Mixed respiratory alkalosis & metabolic acidosis
Sepsis
Liver failure
Mixed respiratory acidosis & metabolic alkalosis
COPD with excessive use of diuretics
Mixed- when compensation is inappropriate
Acid Base imbalance diagnosed
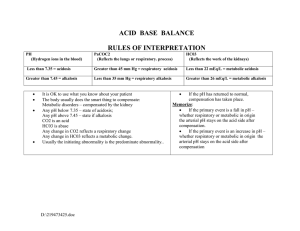
by ABG Analysis
Stepwise approach to ABG Analysis
Determine whether patient is alkalemic or acidemic
using the arterial pH measurement
Determine whether the acid-base disorder is a
primary respiratory or metabolic disturbance based
on the pCO2 and serum HCO3- level
Determine if there is adequate compensation to
identified mixed acid-base disorder
Step 1: Analyze the pH
Normal blood pH is- 7.35 to 7.45
Below 7.35 is acidic
Above 7.45 is alkalotic
Step2: Analyze the CO2
Normal blood CO2 is 35 to 45 mm of Hg
Below 35 is alkalotic
Above 45 is acidic
Step3: Analyze the HCO3
Normal blood HCO3 is 20 to 28 mEq/L
Below 20 is acidic
Above 28 is alkalotic
Step 4: See either the CO2 or
the HCO3 match with the pH
pH
acidic
PCO2 –acidotic
HCO3- alkalotic
Respiratory Acidosis
pH- alkalotic
PCO2- acidic
HCO3- alkalotic
Metabolic Alkalosis
The CO2 is the respiratory component of the ABG
It move in opposite directions to match with pH
↓pCO2
↑ pH
↑ pCO2
↓ pH
The HCO3 is the metabolic component of the ABG.
It move in the same direction to match with pH
↓ HCO3
↓ pH
↑ HCO3
↑ pH
Step 5: Does the CO2 or the HCO3 go the
opposite direction of the pH?
If so, there is compensation by that system
For examplepH – acidotic
CO2- acidotic
HCO3 - alkalotic.
The CO2 matches the pH making the primary acid
‐base disorder respiratory acidosis.
The HCO3 is opposite of the pH and would be
evidence of compensation from the metabolic
system.
Example-1
pH
= 7.30 (7.35)
PaCO2 = 56 (35-45)
HCO3 = 24 (20-28)
ACIDOSIS
ACIDOSIS = Lungs
NORMAL
Respiratory Acidosis (Uncompensated)
Example-2
pCO2 = 62 (35-45)
HCO3 = 35 (20-28)
pH = 7.33(7.35-7.45)
ACIDOSIS
ALKALOSIS
ACIDOSIS
Respiratory Acidosis (compensated )
Step 6: Determine whether the patient’s
compensation is appropriate?
Appropriate compensation
Inappropriate compensation
Simple acid-base disorder
Mixed acid-base disorder
Example-3
pH =7.31 (7.35-7.45)
PaCO2 = 39 (35-45)
HCO3 = 17 (22-26)
ACIDOSIS
NORMAL = lungs
ACIDOSIS = kidneys
Metabolic Acidosis (Uncompensated)
Example-4
pCO2 = 30 (35-45)
HCO3 = 18 (22-26)
pH
= 7.29 (7.35-7.45)
ALKALOSIS
ACIDOSIS
ACIDOSIS
Metabolic Acidosis (compensated )
Appropriate Compensation
METABOLIC ACIDOSIS
Expected PCO2 = 1.5 X (HCO3)+8 ±2
METABOLIC ALKALOSIS
10 mEq/L INCR. IN HCO3 LEADS TO 7 mmHg INNCR. IN PCO2
RESPIRATORY ACIDOSIS
ACUTE:
10 mmHg INCR. IN PCO2 LEADS TO 1 mEq/L INCR. IN
HCO3
CHRONIC: 10 mmHg INCR. IN PCO2 LEADS TO 3.5 mEq/L INCR. IN
HCO3
RESPIRATORY ALKALOSIS
ACUTE:
10 mmHg DECR. IN PCO2 LEADS TO 2 mEq/L DECR. IN
HCO3
CHRONIC: 10 mmHg DECR. IN PCO2 LEADS TO 4 mEq/L DECR. IN
HCO3
Acidemia ( Low pH-7.29 )
Res. Acidosis
( High pco2-65 )
Met. acidosis
( Low HCO3-14)
High Pco2
Low Pco2
Exp. Pco2=
27-31
High HCO3
Exp. HCO3=
AC -30
CHR- 35
Simple met.acidosis
Mixed met. Acidosis
and Res. alkalosis
Low HCO3
Mixed met. Acidosis
and Res. acidosis
Simple Res.acidosis
Mixed Res. Acidosis
and met. acidosis
Mixed Res. Acidosis
and met. alkalosis
Alkalemia ( High pH-7.5 )
Met. Alkalosis
( high HCO3=38)
High Pco2
Low Pco2
Exp. Pco2=52
Res. Alkalosis
( Low pco2 =25 )
Low HCO3
High HCO3
Exp. HCO3=
AC - 18
CHR-16
Simple met.alkalosis
Simple Res.alkalosis
Mixed met. Alkalosis
and Res. alkalosis
Mixed met. Alkalosis
and Res. acidosis
Mixed Res. Alkalosis
and met. acidosis
Mixed Res. Alkalosis
and met. alkalosis
Example-5
pCO2 = 33 (35-45)
HCO3 = 16 (22-26)
pH
=7.21 (7.35-7.45)
ALKALOSIS
ACIDOSIS
ACIDOSIS
Expected Compensation(Pco2)= 1.5 X 16 + 8± 2
= 32 ± 2
= 30-34
So, compensation is appropriate
Simple Metabolic Acidosis (compensated )
Example-6
pCO2 = 45 (35-45)
HCO3 = 17 (22-26)
pH
=7.14 (7.35-7.45)
NORMAL
ACIDOSIS
ACIDOSIS
Expected Compensation(Pco2)= 1.5 X 17 + 8± 2
= 33.5 ± 2
= 31.5-35.5
So, compensation is inappropriate, PCO2 > Expected
Mixed Metabolic and Respiratory Acidosis