Autonomic Nervous System: Sympathetic & Parasympathetic Function
advertisement

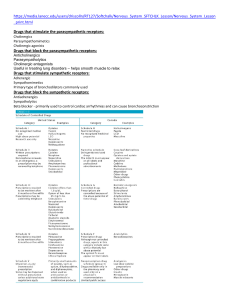
The Function of Sympathetic Neurons - The sympathetic division is adjusted in response to stressful situations, such as trauma, fear, hypoglycemia, cold, and exercise through neuronal (sympathetic nerves) and hormonal (epinephrine). The effects of sympathetic output are: A. Increasing heart rate and blood pressure. (Heart) B. Increasing blood glucose. (Liver, glycogen) C. Increasing blood flow to skeletal muscles and the heart, while diverting it from the skin and internal organs. D. Dilation of the pupils and the bronchioles. (eyes and lungs) E. Decreasing gastro-intestinal motility. (GIT) F. Decreasing urination. (bladder) G. Affecting sexual organs. (genitalia) Fight and flight response The Function of Parasympathetic Neurons — The parasympathetic division: — Acts to oppose or balance the actions of the sympathetic division. — Generally predominates the sympathetic system in ―rest-and-digest‖ situations. A. B. C. D. E. Slows heart rate and decreases blood pressure.. Increases gastrointestinal motility. Protects retina from excessive light. (pupil constriction). Empties the bowel and bladder. Promotes absorption of nutrients. The role of CNS in the control of autonomic function? — It requires sensory inputs from periphery as a feedback for the current state of the body. These inputs are travelled to CNS as a streams of afferent impulses originating in the autonomically innervated structures. CNS vital parts such as hypothalamus, medulla oblongata and spinal cord respond to the stimuli by sending out efferent reflex impulses via ANS. — Afferent impulses are involuntary translated into reflex responses. — Falling the blood pressure innervate pressure-sensitive neurons to send impulses to cardiovascular center in the brain to activate and subsequently activates sympathetic outputs to the heart and vascular system and at the same time decreasing parasympathetic outputs. — On the other hand, emotional feelings are also considered as stimuli of CNS resulting in modifications in the activities of ANS. Introduction The Autonomic Nervous System (ANS) The ANS carries nerve impulses from the CNS to the effector organs by way of two types of efferent neurons: a) The preganglionic neurons: • The cell body of the first nerve is located within the CNS. • They make a synaptic connection with postganglionic in ganglia (an aggregation of nerve cell bodies located in the peripheral nervous system). a) The postganglionic neurons • Originates in the ganglion and terminates on effectors organs. Innervation by the Autonomic Nervous System (ANS) Dual innervation —Most organs in the body are innervated by both divisions of the ANS. —Despite this dual innervation, one system usually predominates in controlling the activity of a given organ. —For example, the vagus nerve is the predominant factor for controlling heart rate. Organs receiving only sympathetic innervations — Some effector organs, such as the adrenal medulla, kidney, pilomotor muscles, and sweat glands, receive innervation only from the sympathetic system. Chemical Signalings Between Cells A. Hormones - Specialized endocrine cells secrete hormones into the bloodstream. - Hormones then widely distribute and exert effects on target cells. B. Local mediators ― Most cells secrete chemicals that act locally on cells in the surrounding environment. ― These chemical signals are rapidly destroyed or removed; therefore they do not enter the blood and are not distributed throughout the body. (e.g., Histamine & Prostaglandins). C. Neurotransmitters ― Communication between nerve cells, and between nerve cells and effector organs, occurs through the release of neurotransmitters from the nerve terminals. ― The neurotransmitters rapidly diffuse across the synaptic cleft, or space (synapse), between neurons and combine with specific receptors on the postsynaptic or target cell. ― All neurotransmitters are too hydrophilic to penetrate the lipid bilayers of target cell plasma membranes. ― The neurotransmitter signal is mediated by binding to specific receptors on the cell surface of target organs. Types of neurotransmitters Acetylcholine (cholinergic agonist) A. Mediates the transmission of nerve impulses of preganglionic to postganglionic nerves in both the sympathetic and parasympathetic nervous systems. B. It is also the neurotransmitter at the adrenal medulla. C. It is also involved in the transmission of signals from the autonomic postganglionic nerves to the effector organs in the parasympathetic system, and a few sympathetic system organs (skin and sweat gland). D. It is involved in the transmission at the neuromuscular junction (the junction of nerve fibers and voluntary muscles) in somatic nervous system. Types of neurotransmitters Norepinephrine and epinephrine (Adrenergic agonist) —Mediates the transmission of nerve impulses from autonomic postganglionic nerves to effector organs in the sympathetic system. Exceptions – Thermoregulatory sweat glands - acetylcholine – Some blood vessels in skeletal muscle. Types of neurotransmitters Signal Transduction in the Effector Cell —Neurotransmitters (signals) bind to receptors (signals dectors and traducers). —Receptors that bind acetylcholine neurotransmitters are : A. Nicotinic receptors (ligand-gated ion channels) B. Muscarinic receptors (G-protein coupled receptors) — Receptors that bind norepinephrine neurotransmitters are : Adrenergic receptors (G-protein coupled receptors) Cholinergic nerve ending Cholinergic Receptors (Cholinoceptors) 1) Muscarinic receptors: ―G protein–coupled receptors. ― Recognize muscarine, an alkaloid that is present in certain poisonous mushrooms. ― Show only a weak affinity for nicotine. ― There are five subclasses of muscarinic receptors. However, only M1, M2, and M3 receptors have been functionally characterized. Cholinergic Receptors (Cholinoceptors) 1) Location of muscarinic receptors: ― ― ― M1 receptors are found on gastric parietal cells. M2 receptors on cardiac cells and smooth muscle. M3 receptors on the bladder, exocrine glands, and smooth muscle. Cholinergic Receptors (Cholinoceptors) 1) Mechanisms of ACh signal transduction: Binding ACh or muscarine to M-R generates signals which are transmitted through different mechanisms: ― M1 receptors (Production of IP3 and DAG second messengers). ― M2 receptors (Gi > decrease cAMP). ― M3 receptors (production PI3 and DAG second messengers). Cholinergic Receptors (Cholinoceptors) 2) Nicotinic receptors: Show only a weak affinity for muscarine. (highest affinity to nicotine Followed by ACh) ― ― Function as Ligand-gated ion channel. ― Nicotine at low concentration stimulates the receptor, whereas nicotine at high concentration blocks the receptor. ― Are located in the CNS, the adrenal medulla, autonomic ganglia, and the neuromuscular junction in skeletal muscles. Nicotinic receptor at NMJ (designated Nm) is different from that at autonomic ganglia (Nn) ― Direct-Acting Cholinergic Agonists ― Cholinergic agonists mimic the effects of ACh by binding directly to cholinoceptors (muscarinic or nicotinic). ― These agents may be broadly classified into two groups: 1) endogenous choline esters, which include Ach and synthetic esters of choline, such as carbachol and bethanechol. 2) naturally occurring alkaloids, such as nicotine and pilocarpine. ― All of the direct-acting cholinergic drugs have a longer duration of action than Ach! WHY? Cholinergic Agents: Direct-acting Choline Esters Acetylcholine Bethanechol Carbachol Methacholine 19 Alkaloids Muscarine Pilocarpine Acetylcholine ― Is a quaternary ammonium (charged) compound > cannot penetrate membranes. Has both muscarinic and nicotinic activity. ― It lacks therapeutic importance !!!! Because of: a) Its multiplicity of actions b) Its rapid inactivation by the cholinesterases. The actions of Ach include: 1- Decrease (negative chronotropy) in heart rate and cardiac output 2- Decrease in blood pressure ― Injection of ACh causes vasodilation and lowering of blood pressure indirectly by activating endothelial M3 receptors. ― This results in the production of nitric oxide from arginine. Acetylcholine ― Gastrointestinal tract: increases salivary secretion and stimulates intestinal secretions and motility. ― Lung: enhances bronchial secretions. ― Genitourinary tract: increases the tone of the detrusor muscle, causing urination. ― Eye: stimulation of ciliary muscle contraction for near vision and in the constriction of the pupil sphincter muscle, causing miosis (marked constriction of the pupil). ― ACh (1% solution) is instilled into the anterior chamber of the eye to produce miosis during ophthalmic surgery. Bethanechol ― Bethanechol is an unsubstituted carbamoyl ester, structurally related to ACh. ― It is not hydrolyzed by AChE due to the esterification of carbamic acid, although it is inactivated through hydrolysis by other esterases. ― It lacks nicotinic actions (due to the addition of the methyl group) but does have strong muscarinic activity. ― Its major actions are on the smooth musculature of the bladder and GI tract. ― It has about a 1-hour duration of action. Bethanechol Therapeutic applications — In urologic treatment, bethanechol is used to stimulate the atonic bladder, particularly in postpartum or postoperative, nonobstructive urinary retention. — Bethanechol may also be used to treat neurogenic atony as well as megacolon. Adverse effects ― Bethanechol causes the effects of generalized cholinergic stimulation . These include sweating, salivation, flushing, decreased blood pressure, nausea, abdominal pain, diarrhea, and bronchospasm. Carbachol ― Carbachol has both muscarinic and nicotinic actions. ― Like bethanechol, carbachol is an ester of carbamic acid and a poor substrate for AChE (longer duration of action than ACh). Carbachol has profound effects on both the cardiovascular and GI systems because of its ganglion-stimulating activity, and it may first stimulate then depress these systems. ― It can cause release of epinephrine from the adrenal medulla by its nicotinic action (nicotinic receptors on adrenal medulla). ― Therapeutic uses ― In the eye as a miotic agent to treat glaucoma by causing pupillary contraction and a decrease in intraocular pressure. ― It is not used systemically because of its high potency, receptor nonselectivity, and relatively long duration of action. ― Adverse effects ― At doses used ophthalmologically, little or no side effects occur due to lack of systemic penetration (quaternary amine). Pilocarpine ― Alkaloid ― Pilocarpine is a tertiary amine and is stable to hydrolysis by AChE. ― Is uncharged and can penetrate the CNS at therapeutic doses. ― Exhibits muscarinic activity and is used primarily in ophthalmology. ― Is one of the most potent stimulators of secretions such as sweat, tears, and saliva. ― The drug is beneficial in promoting salivation in patients with xerostomia resulting from irradiation of the head and neck. ― Sjögren syndrome, which is characterized by dry mouth and lack of tears, is treated with oral pilocarpine. Pilocarpine Therapeutic use in glaucoma Pilocarpine is used to treat glaucoma and is the drug of choice for emergency lowering of intraocular pressure of both open-angle and angle-closure glaucoma. Adverse effects — Can cause blurred vision, night blindness, and brow ache. — Poisoning with this agent is characterized by exaggeration of various parasympathetic effects, including profuse sweating (diaphoresis) and salivation. Major Contraindication to the Use of Muscarinic Agonists Asthma: • Choline esters (muscarinic agonists) can produce bronchoconstriction. In the predisposed patient, an asthmatic attack may be induced. 27 Peptic ulcer. • Choline esters (muscarinic agonists), by increasing gastric acid secretion, may exacerbate ulcer symptoms. Coronary vascular disease. • Choline esters (muscarinic agonists), as a result of their hypotensive effects, can further compromise coronary blood flow. Adverse Effects: Muscarinic Agonists • • • • • • • • Salivation Diaphoresis (perspiration, especially profuse perspiration) Colic GI hyperactivity Nausea and Diarrhea Urinary urgency Headache Loss of accommodation (eye) and miosis Antidote of Direct-Acting Cholinergic Agonists ― Atropine (antimuscarinic) may be administered as an antidote. ― Parenteral atropine, at doses that can cross the blood–brain barrier, is administered to counteract the toxicity of pilocarpine. Indirect-Acting Cholinergic Agonists Anticholinesterase Agents (Reversible) Anticholinesterase Agents (acetylcholinesterase (AChE) inhibitor): 1) Reversible Mechanism of inhibition 2) Irreversible ― Inhibitors of AChE indirectly provide a cholinergic action by preventing the degradation of ACh> accumulation of ACh in the synaptic space. ― These drugs can provoke a response at all cholinoceptors in the body, including both muscarinic and nicotinic receptors. Edrophonium • Is the prototype short-acting AChE inhibitor. • It is rapidly absorbed and has a short duration of action of 10 to 20 minutes due to rapid renal elimination. • It is used in the diagnosis of myasthenia gravis!!!. • Intravenous injection of edrophonium leads to a rapid (due to fast onset of action) increase in muscle strength. • May also be used for reversing the effects of nondepolarizing neuromuscular blockers after surgery. Myasthenia Gravis • Myasthenia Gravis is an auto-immune neuromuscular disorder primarily characterized by muscle weakness and rapid muscle fatigue. • Anticholinesterase drugs provide partial improvement in myasthenia gravis by increasing the amount of acetylcholine available at neuromuscular junctions. • Either restricted to certain muscle groups, particularly those of the eyes (Ocular Myasthenia Gravis), or more generalized (Generalized Myasthenia Gravis), involving multiple muscle groups. 32 5/10/2020 Physostigmine • Is a nitrogenous carbamic acid ester found naturally in plants. • Its duration of action is about 30 min to 2 hours and it is considered an intermediate-acting agent. • Physostigmine (tertiary uncharged amine) can enter and stimulate the cholinergic sites in the CNS. • It is used to stimulate the bladder and GI tract. • Also as an antidote for competitive neuromuscular-blocking agents (such as tubocurarine). • Is also used to manage symptoms of myasthenia gravis. • The effects of physostigmine on the CNS may lead to convulsions when high doses are used. Neostigmine • Is also a carbamic acid ester. • It reversibly inhibits AChE in a manner similar to that of physostigmine. • Unlike physostigmine, neostigmine has a quaternary nitrogen (charged) > more polar, is absorbed poorly from the GI tract, and does not enter the CNS. • • Its effect on skeletal muscle is greater than that of physostigmine, and it can stimulate contractility before it paralyzes. >> used in myasthenia gravis. stimulates the bladder and GI tract. • Has an intermediate duration of action, usually 30 minutes to 2 hours. • Does not cause CNS side effects!!!!! and is not used to overcome toxicity of central-acting antimuscarinic agents such as atropine. • • Neostigmine is contraindicated when intestinal or urinary bladder obstruction is present. t1/2= 30 min to 2 hrs. Demecarium • Is quaternary amine (charged ionized) that is structurally related to neostigmine. • Used in treatment of: a) chronic open-angle glaucoma. b) Accommodative esotropia (because it enhances the cyclotonic effect of parasympathetic neuronal input to the ciliary muscle) Pyridostigmine and ambenonium • Are used in the chronic management of myasthenia gravis?? • Their durations of action are longer than that of neostigmine. • Adverse effects of these agents are similar to those of neostigmine. Tacrine, donepezil, rivastigmine, and galantamine • These drugs are used mainly (first line treatment) in treatment of Alzheimer’s disease. • Alzheimer patients have a deficiency of cholinergic neurons in the CNS. • Tacrine is not commonly used because of its hepatotoxicity. • GI distress is their primary adverse effect. Indirect-Acting Cholinergic Agonists Anticholinesterase Agents (Irreversible) • Organophosphate compounds bind covalently to AChE > long-lasting increase in ACh at all sites where it is released. • Many of these drugs are extremely toxic and were developed by the military as nerve agents (sarin gas). • Toxicity with these agents is manifested as nicotinic and muscarinic signs and symptoms (cholinergic crisis). • Parathion and malathion are used as Organophosphate compounds insecticides. Echothiophate • Is an organophosphate that covalently binds via its phosphate group at the active site of AChE . • Restoration of AChE activity requires the synthesis of new enzyme molecules. • The phosphorylated AChE slowly releases one of its ethyl groups which is called aging, makes it impossible for chemical reactivators, such as pralidoxime, to break the bond between the remaining drug and the enzyme. • Atropine (at high dose) can be used to reverse many of the peripheral and some of the central muscarinic effects. • Can enter the CNS resulting in convulsions. • A topical ophthalmic solution of the drug is available for the treatment of open-angle glaucoma. • However, echothiophate is rarely used due to its side effect profile, which includes the risk of causing cataracts. • Long duration of action (1 week). Reactivation of acetylcholinesterase (antidote) • Pralidoxime (2-PAM) can reactivate inhibited AChE If given before aging of the AChE >>> it is used as antidote of organophosphate toxicity. • It reverses the effects of organophosphates in periphral tissues, but not the CNS effects!!!! Because it can’t cross the blood brain barrier. (Atropine is administrated with pralidoxime to decrease the effect of organophosphate compounds in CNS. • Pralidoxime is a weak AChE inhibitor and, at higher doses, may cause side effects similar to other AChE inhibitors. • It cannot overcome toxicity of reversible AChE inhibitors (e.g., physostigmine). Cholinergic Antagonists Cholinergic Antagonists —Cholinergic antagonist is a general term for agents that bind to cholinoceptors (muscarinic or nicotinic) and prevent the effects of Ach and other cholinergic agonists. Cholinergic antagonist Muscarinic antagonist Nicotinic antagonist Ganglionic blockers neuromuscular blockers Cholinergic Antagonists Sites of actions of cholinergic antagonists Muscarinic antagonist • The most clinically useful cholinergic antagonist are selective blockers of muscarinic receptors. • They are commonly known as antimuscarinic agents or parasympatholytics (The effects of parasympathetic innervation are, interrupted, and the actions of sympathetic stimulation are left unopposed). • These drugs block the few exceptional sympathetic neurons that are cholinergic, such the sweat glands. • Because do not block nicotinic receptors, the antimuscarinic drugs have little or no action at skeletal neuromuscular junctions or autonomic ganglia. Atropine • Tertiary amine belladonna alkaloid (readily absorbed). • High affinity for muscarinic receptors. • Binds competitively and prevents ACh from binding to muscarinic receptors. • Acts both centrally (cross blood brain barrier) and peripherally. • The greatest inhibitory effects are on bronchial tissue and the secretion of sweat and saliva Eye (Beautiful lady!!!!) Atropine • Mydriasis (dilation of the pupil) • Unresponsiveness to light • Cycloplegia (inability to focus for near vision). May cause problems with driving or operating machinery. • Patients may experience Photophobia: sensitivity to light and may want to wear dark glasses or sunglasses. • In patients with angle-closure glaucoma, intraocular pressure may rise dangerously (contraindicated). Atropine Gastrointestinal (GI) • Antispasmodic to reduce activity of the GI tract. • Most potent antispasmodic drugs available. • Treatment of Irritable bowel disease • May cause constipation Cardiovascular • Atropine produces divergent effects on the cardiovascular system, depending on the dose: a) At low doses, the predominant effect is a slight decrease in heart rate. This effect results from blockade of the M1 receptors on the inhibitory prejunctional (or presynaptic) neurons, thus permitting increased ACh release. b) Higher doses of atropine cause a progressive increase in heart rate by blocking the M2 receptors on the sinoatrial node. Atropine Secretions • Dryness of the mouth (xerostomia). • Decreases sweating and tearing. • Is used as an antisecretory agent to block secretions in the respiratory tract prior to surgery. Genitourinary • Relaxed detrusor muscle • Increased constriction of internal sphincter Result: urinary retention Antidote for cholinergic agonists • Atropine is used for the treatment of organophosphate (insecticides, nerve gases) poisoning, of overdose of clinically used anticholinesterases such as physostigmine. Atropine Adverse effects • Dry mouth, blurred vision, ―sandy eyes dry red irritant eyes,‖ tachycardia, urinary retention, and constipation. • Effects on the CNS include restlessness, confusion, hallucinations, and delirium, which may progress to depression, collapse of the circulatory and respiratory systems, and death. • Physostigmine, may be used to overcome atropine toxicity.!!!! Why?? • The drug may be dangerous in children, because it may dangerously elevate their body temperature. • Anticholinergics may lead to higher risk for heat stroke due to effects on heat-regulating mechanisms. Scopolamine • Tertiary amine belladonna alkaloid. • Has greater action on the CNS and a longer duration of action as compared to atropine. • Is effective anti–motion sickness (i.e., it can treat the condition once it occurs but to less extent comparing with its prophylactic effect). • In contrast to atropine, scopolamine produces sedation, but at higher doses, it can produce excitement. • The therapeutic use of scopolamine is limited to prevention of motion sickness, postoperative nausea and vomiting, GI and urinary spasms. • For motion sickness, it is available as a topical patch that provides effects for up to 3 days. • May produce euphoria and is susceptible to abuse. Hyoscine • Semi- synthetic derivative of scopolamine. • Works more peripherally on the GI tract than centrally. • Is effective Antispasmodic. Available in Jordan Ipratropium and tiotropium Available in Jordan • Are quaternary derivatives of atropine, do not enter the systemic circulation or the CNS. • Are approved as bronchodilators for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD). • Ipratropium is also used in the acute management of bronchospasm in asthma. • Both agents are delivered via inhalation. • • Tiotropium is administered once daily, a major advantage over ipratropium, which requires dosing up to four times daily. Ipratropium (Atrovent) has an advantage in asthma compared to atropine because: • • • Ipratropium has no significant CNS effects. Inhaled Ipratropium (a quaternary, charge molecule) has limited or no systemic effects. Ipratropium is as effective as -adrenergic agonists in COPD. Tropicamide and cyclopentolate • These agents are used as ophthalmic solutions for mydriasis and cycloplegia. Benztropine and trihexyphenidyl • They are used as adjuncts antiparkinsonian agents to treat Parkinson’s disease and antipsychotic induced extrapyramidal symptoms. Available in Jordan Darifenacin, fesoterodine, oxybutynin • These synthetic atropine-like drugs are used to treat overactive bladder. • Oxybutynin is available as a transdermal system (topical patch), which is better tolerated because it causes less dry mouth than oral formulations. • The overall efficacies of these antimuscarinic drugs are similar. Prifinium • • Is an anticholinergic with selective action on the gastro-intestinal and urinary tracts. It inhibits pain from spasm or hypermotility of these organs. Available in Jordan Cholinergic Blocking Agents Side effects, including: Constipation Tachycardia Tremors Confusion Hallucinations Sedation Urinary retention Hot, dry skin Fever CNS depression (occurs with large doses) 55 5/10/2020 Ganglionic Blockers Ganglionic Blockers • Specifically act on the nicotinic receptors of both parasympathetic and sympathetic autonomic ganglia: A) No selectivity toward the parasympathetic or sympathetic ganglia B) Not effective as neuromuscular blockers • Are rarely used therapeutically. • Depending on the mechanism of action it can be subdivided into: a) Depolarizing b) Non-depolarizing Nicotine • A component of cigarette smoke. • Is a depolarizing ganglionic blocker. • Depending on the dose: Low dose: nicotine depolarizes autonomic ganglia, resulting first in stimulation and then in paralysis of all ganglia. - Enhanced release of dopamine and norepinephrine > >associated with pleasure, appetite suppression (lower body weight), increased blood pressure, cardiac rate and peristalsis and secretions in the GI tract. Higher doses, the blood pressure falls and activity in both the GI tract and bladder musculature ceases because of ganglionic blockade . Mecamylamine • Produces competitive nicotinic blockade of the ganglia. • Antihypertensive agent (emergeny) in sever cases. • Is well absorbed from the gastrointestinal tract and crosses the blood-brain barrier. • like most ganglionic blockers, is more often used now as a research tool. • Mecamylamine is also sometimes used as an anti-addictive drug to help people stop smoking tobacco (smoking cessation). • side effects, including drowsiness, postural hypotension and constipation. Neuromuscular Blockers Neuromuscular-Blocking Agents • Block cholinergic transmission between motor nerve endings and the nicotinic receptors on the skeletal muscle. • Act either as: A) Non- depolarizing type(Antagonists) Or B) Depolarizing type (Agonists) at the receptors on the endplate of the NMJ. • Are clinically useful during surgery to facilitate trachea intubation and provide complete muscle relaxation at lower anesthetic doses (safe). A. Nondepolarizing blockers • Curare, which native South American hunters used to paralyze prey. • The first detection of curare has been followed by the development of the drug called tubocurarine. However, the latter has been replaced by safer agents, such as pancuronium, rocuronium , and vecuronium. • Are injected intravenously or occasionally intramuscularly since they are not effective orally. This is because their structure (charged and bulky) that prevents their absorption from the gut. Also, they penetrate membranes very poorly and do not cross BBB. A. Nondepolarizing blockers • Competitively block ACh at the nicotinic receptors without stimulating it. • Prevent depolarization of the muscle cell membrane and inhibit muscular contraction. • Not all muscles are equally sensitive to blockade by competitive agents. • Small, rapidly contracting muscles of the face and eye are most susceptible and are paralyzed and lastly the muscle of respiration. B. Depolarizing agents • Work by depolarizing the plasma membrane of the muscle fiber, similar to the action of ACh. • However, these agents are more resistant to degradation by acetylcholinesterase and can thus more persistently depolarize the muscle fibers. • Succinylcholine is the only depolarizing muscle relaxant. Succinylcholine • Attaches to the nicotinic receptor opening the associated Na+ channel and consequently depolarizing the receptor (Phase I) > > leads to a transient twitching of the muscle (fasciculations). • The depolarizing agent persists at high concentrations in the synaptic cleft, remaining attached to the receptor for a longer time (because of less inactivation by cholisterase)and providing constant stimulation of the receptor. • Continued binding of the depolarizing agent renders the receptor incapable of transmitting further impulses >> gradual repolarization as the sodium channel closes or is blocked >> resistance to depolarization (Phase II) and paralysis. • Because of its rapid onset of action, succinylcholine is useful when rapid endotracheal intubation is required during the induction of anesthesia. Succinylcholine Adverse effects • 1) Hyperthermia • Is overomed by administration of dantrolene , which blocks release of Ca 2+ from the sarcoplasmic reticulum of muscle cells, thereby reducing heat production and relaxing muscle tone. 2) Apnea (due to paralysis of the diaphragm) 3) Hyperkalemia (increases potassium release from intracellular stores to the plasma) Adrenergic Agonists Adrenergic Agonists • Activate receptors (adrenoceptors) that are stimulated by norepinephrine (noradrenaline) or epinephrine (adrenaline). • Adrenergic drugs act on adrenergic receptors, located either presynaptically on the neuron (indirect-acting agonist) or postsynaptically on the effector organ (direct-acting agonist). • Adrenergomimetics and sympathomimetics. Neurotransmission at adrenergic neurons Neurotransmission involves the following steps: 1) Synthesis Tyrosine is transported by a carrier into the adrenergic neuron>> It is hydroxylated to dihydroxyphenylalanine (DOPA) >> decarboxylated to form dopamine. 2) Storage Dopamine is transported into vesicles by transporter system (blocked by reserpine) >>Dopamine is hydroxylated to form norepinephrine. 3) Release Drug guanethidine block this release 4) Binding of norepinephrine to a receptor Norepinephrine released from the synaptic vesicles 5) Removal of the neurotransmitter in the synaptic cleft Norepinephrine may 1) diffuse out of the synaptic space and enter the systemic circulation 2) be metabolized to inactive metabolites by catechol-O-methyltransferase (COMT) in the synaptic space 3) undergo reuptake back into the neuron (primary mechanism in the termination of norepinephrine and involves a sodium-chloride (Na+/Cl-)-dependent norepinephrine transporter). 6) Potential fates of recaptured norepinephrine (continue). Continue..... 6) Potential fates of recaptured norepinephrine. • once NE reenters the adrenergic neuron, 1. It may be taken up into synaptic vesicles via the transporter system and be sequestered for release by another action potential. 2. It may persist in a protected pool in the cytoplasm. 3. Alternatively, norepinephrine can be oxidized by monoamine oxidase (MAO) present in neuronal mitochondria. Adrenergic receptors Basis of their responses to the adrenergic agonists epinephrine, norepinephrine, and isoproterenol. β adrenergic receptors α adrenergic receptors Rank order of potency and affinity is epinephrine ≥ norepinephrine >> isoproterenol. Rank order of potency is isoproterenol > epinephrine > norepinephrine. α1 adrenergic receptors α2 adrenergic receptors • Have a higher affinity for phenylephrine. • Have a higher affinity for drug clonidine. • Present on the postsynaptic membrane of • located primarily on sympathetic • presynaptic nerve endings. • the effector organs. • Involving constriction of smooth muscle. • Second messengers are (IP3) and (DAG). • Inhibitory receptors of norepinephrine release through reducing intracellulrar cAMP. β1 • β2 Heart Vessels of skeletal muscle Lung β3 • Lipolysis. • Have effects on the detrusor muscle of the bladder. Results in activation of adenylyl cyclase and increased cAMP concentrations within the cell Characteristics of Adrenergic Agonists Catecholamines • Sympathomimetic amines epinephrine, norepinephrine, isoproterenol, and dopamine are called catecholamines. • These compounds share the following properties: 1. High potency to α or β receptors. 2. Rapid inactivation: Catecholamines are metabolized by COMT and MAO>>> catecholamines have only a brief period (very short) of action. 3. Poor penetration into the CNS because they are polar and poorly absorbed through the GI tract. • Characteristics of Adrenergic Agonists Noncatecholamines • lack the catechol hydroxyl groups (polar groups)>> permits greater access to the CNS. • have longer halflives and prolonged duration of action!!!!! because they are not inactivated by COMT and MAO. • These include phenylephrine, ephedrine, and amphetamine. Adrenergic Agonists Depending on the mechanism of action Direct acting Indirect acting Stimulate adrenergic receptor directly by Direct binding to adrenergic receptors without interacting with the presynaptic neuron. May block the uptake of norepinephrine (uptake blockers) or are taken up into the presynaptic neuron and cause the release of norepinephrine. Mixed acting Has the capacity both to stimulate adrenoceptors directly and to release norepinephrine from the adrenergic neuron. Direct-Acting Adrenergic Agonists Direct-Acting Adrenergic Agonists Epinephrine • Is catecholamine commonly used in therapy. • Interacts with both α and β receptors (Biological response is wide!!!). At low doses, it causes vasodilatation due to β stimulation on the vascular system, At high doses, causes vasoconstriction due to α receptor stimulation which is strong. • In the adrenal medulla, norepinephrine is methylated to yield epinephrine. • The adrenal medulla releases about 80% epinephrine and 20% norepinephrine directly into the circulation. Epinephrine Cardiovascular • Increase in systolic blood pressure, coupled with a slight decrease in diastolic pressure. WHY?!!! • Strengthens the contractility of the myocardium (positive inotrope) and increases its rate of contraction (positive chronotrope) due to β1 receptor activation in the heart. • Activates β1 receptors on the kidney to cause renin release. • Dilates vessels going to the liver and skeletal muscle due to β2 receptor activation. Treatment: • Epinephrine may be used to restore cardiac rhythm in patients with cardiac arrest because positive inotrope and chronotrope effect of epinephrine. Epinephrine Respiratory • Epinephrine is the primary drug used in the emergency treatment of respiratory conditions and type I hypersensitivity reactions (including anaphylaxis) in response to allergens. WHY!!! A. Bronchodilation (β2 receptor) B. Dereasing of histamine release. Hyperglycemia (Increase blood sugar level): • Because of: A. increased glycogenolysis in the liver (β2 receptor) B. Increased release of glucagon (β2 receptor) C. Decreased release of insulin (α2 receptor) . Lipolysis Epinephrine Adverse effects 1. Epinephrine can produce adverse CNS effects that include anxiety, fear, tension, headache, and tremor. 2. cardiac arrhythmias. 3. Pulmonary edema. 4. Epinephrine may have enhanced cardiovascular actions in patients with hyperthyroidism. 5. Hyperglycemia. Norepinephrine • Stimulate mainly α1 receptors, and has weak effect on β2 receptors. As a result causes: 1. Rise in peripheral resistance due to intense vasoconstriction of most vascular beds, including the kidney >>> Both systolic and diastolic blood pressures increase. 2. The weak β2 activity of norepinephrine also explains why it is not useful in the treatment of asthma or anaphylaxis. • It produces a reflex bradycardia!!! Norepinephrine increases blood pressure, and this stimulates the baroreceptors, inducing a rise in vagal activity and produces a reflex bradycardia, which is sufficient to counteract the local actions of norepinephrine on the heart. Norepinephrine is given IV for rapid onset of action but it has short duration???. Norepinephrine Therapeutic uses A. Norepinephrine is used to treat shock, because it increases vascular resistance and, therefore, increases blood pressure. B. It has no other clinically significant uses. Adverse effects • These are similar to epinephrine!!!!!. • In addition, norepinephrine is a and may cause blanching ()إبيضاضand sloughing إنسالخof skin along an injected vein (potent vasoconstrictor) . • Impaired circulation from norepinephrine may be treated with the α receptor antagonist phentolamine. Isoproterenol • Stimulates both β1- and β2-adrenergic receptors: • Stimulation of the heart, increasing heart rate, contractility, and cardiac output. • Dilates the arterioles of skeletal muscle, resulting in decreased peripheral resistance. Results: • It may increase systolic blood pressure slightly, but it greatly reduces mean arterial and diastolic blood pressures. • Is a potent bronchodilator WHY? • Due to its nonselectivity, the use of isoproterenol has largely been replaced with other drugs. Dopamine The effect of dopamine depends on its dose: • At higher doses, it causes vasoconstriction by activating α1 receptors (rises blood pressure). • At intermediate doses, it stimulates β1 cardiac receptors having both positive inotropic and chronotropic effects (cardiogenic). • At low doses, it binds to D1 and D2 dopaminergic receptors in the peripheral mesenteric and renal vascular beds, where binding of dopamine produces vasodilation. thereby increasing blood flow to the kidneys and causes diuresis making it a far better option than norepinephrine which diminishes kidney blood supply. (IMPORTANT). • Dopamine is clinically useful in the treatment of shock (septic shock), in which significant increases in sympathetic activity might compromise renal function. Fenoldopam • An agonist of peripheral dopamine D1 receptors. • vasodilator. • It is used as a rapid-acting vasodilator to treat severe hypertension in hospitalized patients, acting on coronary arteries, kidney arterioles, and mesenteric arteries. Dobutamine • Is a β1 receptor agonist. • It increases cardiac rate and output with few vascular effects (inotropic). • Is used to increase cardiac output in acute heart failure. • Should be used with caution in atrial fibrillation. Oxymetazoline • Stimulates both α1- and α2-adrenergic receptors. • Is found in many over-the-counter short-term nasal spray decongestants, as well as in ophthalmic drops for the relief of redness of the eyes associated with swimming, colds, and contact lenses. Because of directly stimulates α receptors on blood vessels supplying the nasal mucosa and conjunctiva, thereby producing vasoconstriction and decreasing congestion. • Rebound congestion and dependence are observed with long-term use. Phenylephrine • Binds primarily to α1 receptors >>> raises both systolic and diastolic blood pressures. • Is not catechol. • It has no effect on the heart itself but, induces reflex bradycardia. • The drug is used to treat hypotension and to induce mydriasis (ophthalmic solutions). • Acts as a nasal decongestant when applied topically or taken orally. • Phenylephrine has replaced pseudoephedrine in many oral decongestants, since pseudoephedrine has been misused to make methamphetamine. Clonidine • Is an α2 agonist that is used for the treatment of hypertension. • Acts centrally on presynaptic α2 receptors to produce inhibition of sympathetic vasomotor centers. • It can also be used to minimize the symptoms that accompany withdrawal from opiates, tobacco smoking, and benzodiazepines (it acts centrally to produce inhibition of sympathetic vasomotor centers, decreasing sympathetic outflow to the periphery). Mirabegron • Is a β3 agonist that relaxes the detrusor smooth muscle and increases bladder capacity. • It is used for patients with overactive bladder. Albuterol, Salbutamol, and terbutaline • Are short-acting β2 agonists used primarily as bronchodilators. • Albuterol is the short-acting β2 agonist of choice for the management of acute asthma symptoms. Salmeterol and formoterol • Are long acting β- agonists that are β2 selective. • Provides sustained bronchodilation. • Are the agents of choice for treating nocturnal asthma in symptomatic patients taking other asthma medications. • β2 -receptor agonists should not be used in excess. Death has been reported in overuse of these medications. Indirect-Acting Adrenergic Agonists Amphetamine • Increases the release of catecholamines such as dopamine and norepinephrine from nerve terminals >>Central stimulatory action >> drug abuse. • Increase blood pressure significantly by α1 agonist action on the vasculature, as well as β1-stimulatory effects on the heart. • It is used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy, due to the CNS stimulatory effect of amphetamine. Mixed-Action Adrenergic Agonists Mixed-Action Adrenergic Agonists • Ephedrine and pseudoephedrine are mixed-action adrenergic agents: 1. They release stored norepinephrine from nerve endings Thus, a wide variety 2. Directly stimulate both α and β receptors. of adrenergic actions • Have a long duration of action??? WHY!! Because they are not catechols and are poor substrates for COMT and MAO. • Ephedrine and pseudoephedrine have excellent absorption orally and penetrate into the CNS (not catechols). • Ephedrine and pseudoephedrine have excellent absorption orally and penetrate into the CNS, but pseudoephedrine has fewer CNS effects. • Are plant alkaloids Mixed-Action Adrenergic Agonists Ephedrine : A. Raises systolic and diastolic blood pressures by vasoconstriction and cardiac stimulation and can be used to treat hypotension. B. Produces bronchodilation, but it is less potent and slower acting than epinephrine or isoproterenol so it is rarely used to relief bronchoconstriction. C. Ephedrine produces a mild stimulation of the CNS, increases alertness, decreases fatigue, and prevents sleep >>It is also addictive and subject to abuse. • Ephedrine-containing herbal supplements (mainly ephedra-containing products) were banned by the U.S. Food and Drug Administration because of life-threatening cardiovascular reactions. Pseudoephedrine: Is primarily used orally to treat nasal and sinus congestion. Has been illegally used to produce methamphetamine. Adrenergic Antagonists Adrenergic Antagonists (sympatholytics) α-Adrenergic Blocking Agents Non selective α-Blocking (α1 and α2) noncompetitive antagonist •Phenoxybenzamine •Long duration competitive antagonist •Phentolamine •Short duration Selective α1-Blocker α1B-Blocker •Prazocin •Hypertension Selective α2-Blocker •Yohimbine •Increases blood pressure α1A-Blocker •Alfuzocin •BPH β -Adrenergic Blocking Agents α-Adrenergic Blocking Agents α-Adrenergic Blocking Agents • Block α adrenoceptors profoundly affect blood pressure >> resulting in decreased peripheral vascular resistance. • This induces a reflex tachycardia resulting from the lowered blood pressure. • Reverse the α agonist actions of epinephrine. • α1-blockers may cause nasal congestion and orthostatic hypotension. • Blocking α receptors in the ejaculatory ducts, α1 antagonists may cause inhibition of ejaculation. Phenoxybenzamine • Is nonselective and irreversible for both α1 and α2 receptors antagonist. • Is used in the treatment of pheochromocytoma: a catecholamine-secreting tumor of cells derived from the adrenal medulla. • Is sometimes effective in treating Raynaud disease (excessively reduced blood supply) and frostbite. • • • • • • Adverse effects: Postural hypotension Reflex tachycardia Nasal congestion Nausea, and vomiting. It may inhibit ejaculation. Phentolamine • Competitive blocker of α1 and α2 receptors. • Lasts for approximately 4 hours (shorter duration than phenoxybenzamine). • Used for the short-term management of pheochromocytoma. • It is also used locally to prevent dermal necrosis following extravasation of norepinephrine. Prazosin, terazosin, doxazosin, tamsulosin, and alfuzosin • Prazosin, terazosin, and doxazosin are selective competitive blockers of the α1 receptor. • They are more useful than non-selective α blockers in the treatment of hypertension. !!!!!! Why? ( α2 inhibition) • Tamsulosin and alfuzosin are examples of other selective α1 antagonists indicated for the treatment of benign prostatic hyperplasia (BPH)!!! • Tamsulosin has the least effect on blood pressure because it is more selective for α1A receptors in the prostate and bladder. Yohimbine • Is a selective competitive α2-blocker. • It is found as a component of the bark of the yohimbe tree. • Is used as a sexual stimulant and in the treatment of erectile dysfunction (insufficient evidence). • Works at the level of the CNS to increase sympathetic outflow to the periphery. • It is contraindicated in cardiovascular disease because it may worsen these conditions. β-Adrenergic Blocking Agents β-Adrenergic Blocking Agents • All of the clinically available β-blockers are competitive antagonists. • Nonselective β-blockers act at both β1 and β2 receptors • Cardioselective β antagonists primarily block β1 receptors. • Although all β-blockers lower blood pressure, they do not induce postural hypotension, because the α adrenoceptors remain functional. • β-Blockers are effective in treating: • Hypertension, angina, cardiac arrhythmias, myocardial infarction, heart failure, hyperthyroidism, glaucoma and prophylaxis of migraine headaches. Propranolol • Is the prototype β-adrenergic antagonist. • A nonselective β antagonist. Cardiovascular • Diminishes cardiac output, having both negative inotropic and chronotropic effects >> bradycardia >> workload, and oxygen consumption are decreased, and these effects are useful in the treatment of angina. • It directly depresses sinoatrial and atrioventricular nodal activity >> treatment of and arrhythmias. Propranolol Peripheral vasoconstriction • Nonselective blockade of β receptors prevents β2-mediated vasodilation in skeletal muscles >>> increasing peripheral vascular resistance. • In patients with hypertension, total peripheral resistance returns to normal or decreases with long term use of propranolol. • There is a gradual reduction of both systolic and diastolic blood pressures in hypertensive patients !!! possibly because of reduced renin release and effects of beta-blockade on central and peripheral nervous systems (chronic effect on hypertension is better than acute treatment). • Does not reduce blood pressure in people with normal blood pressure. Propranolol Bronchoconstriction • Blocking β2 receptors in the lungs causes contraction of the bronchiolar smooth muscle >>> are contraindicated in patients with COPD or asthma. Disturbances in glucose metabolism • Decreases glycogenolysis and decreased glucagon secretion (Blocking β2 ). • If propranolol is given to a diabetic patient receiving insulin >>> careful monitoring of blood glucose is essential, because pronounced hypoglycemia may occur after insulin injection. Propranolol Migraine • Is effective in reducing migraine episodes when used prophylactically. • It is one of the more useful β-blockers for this indication, due to its lipophilic nature that allows it to penetrate the CNS. • Timolol is the other β-blocker indicated for migraine prophylaxis. Hyperthyroidism • Propranolol and other β-blockers are effective in blunting the widespread sympathetic stimulation that occurs in hyperthyroidism. Propranolol Sexual impairment • Ejaculation in the male is mediated through α-adrenergic activation. • β-blockers do not affect ejaculation . • Some men do complain of impaired sexual activity. • The reasons for this are not clear and may be independent of β receptor blockade. Metabolic disturbances • Lipases in fat cells are activated mainly by β2 and β3 receptor stimulation, leading to the metabolism of triglycerides into free fatty acids. Patients administered nonselective β-blockers have: A. Increased low density lipoprotein (―bad‖ cholesterol) B. Increased triglycerides C. Reduced high-density lipoprotein (―good‖ cholesterol). • These effects may be less profound with cardioselective agents!!!! WHY? . Propranolol CNS effects • Propranolol is lipophilic >>> enters CNS >> has numerous CNS-mediated effects, including depression, dizziness, lethargy, fatigue, weakness, visual disturbances, hallucinations, short-term memory loss, emotional lability, and vivid dreams. • Fewer CNS effects may be seen with more hydrophilic β-blockers (e.g., atenolol)!!!! WHY? since they do not cross the blood–brain barrier as readily. Nadolol and timolol • Nonselective β antagonists >> block β1- and β2-adrenoceptors. • Are more potent than propranolol. • Nadolol has a very long duration of action. • Timolol is used topically in the treatment of chronic open-angle glaucoma and, occasionally, for systemic treatment of hypertension. Acebutolol, atenolol, betaxolol, bisoprolol, esmolol, metoprolol and nebivolol • Cardioselectivity of β-blockers: Selective β1 antagonists….. is most pronounced at low doses and is lost at high doses. These drugs lower blood pressure in hypertension and increase exercise tolerance in angina. These agents are also first-line therapy for chronic stable angina. Atenolol, betaxolol, bisoprolol, esmolol, metoprolol and nebivolol Block the β1 receptors (selective) and low effect on β2 receptor: 1. Minimize the unwanted bronchoconstriction (β2 effect) in asthma and COPD. 2. Have fewer effects peripheral resistance and carbohydrate metabolism than non-selective β blockers!!!!! WHY? (β2 effect on sugar and lipid metabolism) 3. Coldness of extremities (Raynaud phenomenon), a common side effect of βblockers, is less frequent. • Esmolol has a very short half-life. Acebutolol and pindolol • Antagonists with partial agonist activity (intrinsic sympathomimetic activity (ISA). • Acebutolol (β1-selective antagonist) and pindolol (nonselective β-blocker). • β-blockers with ISA minimize the disturbances of lipid and carbohydrate metabolism that are seen with other β-blockers. • Therapeutic use in hypertension • β-blockers with ISA are effective in hypertensive patients with moderate bradycardia.!!!!! • β-blockers with ISA are not used for stable angina or arrhythmias due to their partial agonist effect. Labetalol and carvedilol • Antagonists of both α and β adrenoceptors. • They contrast with the other β-blockers that produce initial peripheral vasoconstriction. Therefore, they are useful in treating hypertensive patients for whom increased peripheral vascular resistance is undesirable. • Labetalol is employed as an alternative to methyldopa in the treatment of hypertension during pregnancy. • Carvedilol is used for the treatment of heart failure. • Intravenous labetalol is also used to treat hypertensive emergencies, because it can rapidly lower blood pressure. • Adverse effects: Orthostatic hypotension and dizziness are associated with α1 blockade. Drugs Affecting Neurotransmitter Release or Uptake • Reserpine • Guanethidine. • Take alook on Figure 7.12 (IMPORTANT)