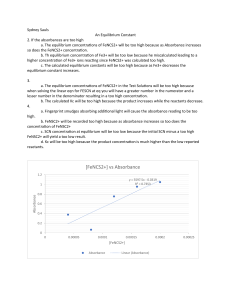
Sarah Kovach May 19, 2019 Dr. Harvey Chemistry 102 lab Title: Equilibria: Measuring on Equilibrium Constant using Spectrophotometry Purpose: The purpose of this experiment is to observe the concentration of Fe3+ and SCN- and measure the equilibrium constant. Equilibrium constant can help us calculate how much of the substance is needed to find equilibrium. A large k value will have the products favored in the reaction. A small k will have reactants favored. A saturated solution will not shift to the right regardless of how much reactant you apply to the solution. The equilibrium can be calculated if we know the concentration amounts of products and reactants. Kp is used for partial pressure of gases to find the ratio of the constant. The general reaction is aA+bB=cC+dD with Kc=[C]c[D]d/[A]a[B]b or substitute partial pressure is such as p[A]a for [A]a. The brackets indicate molarity and the p stands for partial pressure. Iron(III) ion reacts with Thiocyanate ion to produce Thiocyanatoiron (III). The reaction is represented by the following equation: Fe3+ + SCN– <—-> Fe(SCN)2+. The reading from the spectrophotometer, will give a value that needs the conversion factor from the equations ratio to calculate the absorbance of Fe(SCN)2+ at different concentrations. Part 1 determined the limiting reactant in the solution to calculate Fe(SCN)2+. From these values absorbance and Fe(SCN)2+ was used to create a graph that was increasing with absorbance. The absorbed value and linear treadling calculation helped us calculate the molarity for each Fe(SCN)2+ solution. The concentrations for Fe3+ and SCN- were calculated from taking the molarity divided by the total volume. The initial concentration was subtracted from the equilibrium of Fe(SCN)2+ to create new values for the concentration at equilibrium for [Fe3+] and [SCN-]. Lastly, Kc was calculated from the equilibrium constants that were inputted into the equilibrium expression. Conclusion: Water and solids are not included in the equilibrium constant because they do not affect the reactant value at equilibrium. Pure solids and liquids are excluded from this expression because they concentrations remain constant in the reaction. The Kc in this particular experiment favored products because the number is much larger than 1. B-1 through B-5 are different values because they are calculated from different solution concentrations. The results varied a lot from each other. These values explain how the reaction is moving forward and shows how fast the reaction will occur to reach equilibrium. Since A-1 through A-7 had absorbance values and concentration a Kc could be calculated from the reactions stoichiometry. A higher concentration in the solution will have a higher absorption value with less photons making it through the solution tube in the spectrophotometer. According to Le Chatelier's principle, if the reaction was exothermic the Kc would decrease at higher temperature because the reactants are favored. The Kc can tell us if the reaction is moving forward or backward and this value can be compared by the other calculated values to tell how fast the solution is reacting. Calculated values became greater than their previous value which also explains why the graph has a positive slope. As the solution is more concentrated, the light absorbed becomes a greater value. My calculated values were similar to a straight line, and the graph’s R^2 value was 9.97E-01 accuracy. My average Kc value was 15.50. Error could occur from beakers that were not clean, diluting a solution too much or too little, and not reading the burette and beakers accurately.