GCSE Chemistry: Separating Mixtures - Filtering & Distillation
advertisement
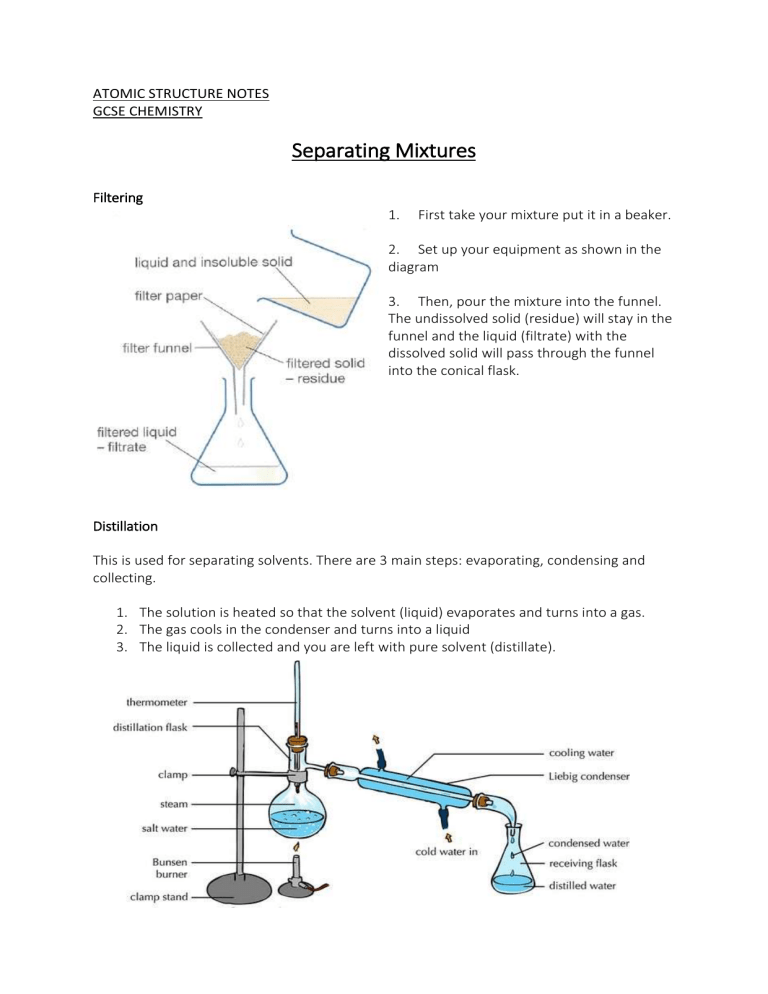
ATOMIC STRUCTURE NOTES GCSE CHEMISTRY Separating Mixtures Filtering 1. First take your mixture put it in a beaker. 2. Set up your equipment as shown in the diagram 3. Then, pour the mixture into the funnel. The undissolved solid (residue) will stay in the funnel and the liquid (filtrate) with the dissolved solid will pass through the funnel into the conical flask. Distillation This is used for separating solvents. There are 3 main steps: evaporating, condensing and collecting. 1. The solution is heated so that the solvent (liquid) evaporates and turns into a gas. 2. The gas cools in the condenser and turns into a liquid 3. The liquid is collected and you are left with pure solvent (distillate). Evaporation - Used to separate soluble solid from a liquid (e.g. salt and water). The solution is heated so that the liquid evaporates, and leaves dissolved solid behind. 1. 2. 3. 4. 5. 6. 7. Crush Rock salt using a mortar and pestle Make sure no salt is trapped in the rocks. Put the crushed salt in a beaker and add water. Stir the mixture to help the salt dissolve. Filter the mixture using the information above. Insoluble bits of rock should be left in the filter paper. Evaporate the filtrate in an evaporating basin to get the salt. The water should have evaporated.




