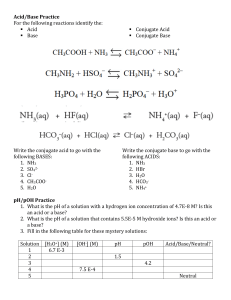
Acids and Bases Name: ___________________________________ Due April 1, 2020 1. A Brønsted acid is a(n) _____. A) proton donor B) hydroxide ion donor C) proton acceptor D) electron donor 2. A Brønsted base _____. A) is a cation B) does not possess a lone pair of electrons C) possesses a lone pair of electrons D) is always an Arrhenius base 3. The conjugate base of formic acid, HCOOH, is _____. A) OH – B) CH3 COO – C) HCOO – D) CO22– 4. In the reaction , _____. A) B) C) D) HPO42– is a conjugate acid HPO42– is a conjugate base NH3 is an acid NH4+ is a conjugate base 5. Which of the following is correct relating [H+] and [OH–] in solution at 25C? A) [H+] + [OH–] = 1 10–14 B) [H+] + [OH–] = 14.0 C) [H+] [OH–] = 1 1014 D) [H+] [OH–] = 1 10–14 6. pH is_______ A) log [H+] B) – log 1/[H+] C) -log [H+] D) 1/log[H+] 7. The equation relating pH and pOH is _____. A) pH pOH= 14 B) pH pOH = 10–14 C) pH + pOH = 14 D) pH + pOH = –14 Page 1 8. The concentration of OH– ions in a 1.4 10–3 M HCl solution is _____. A) 7.1 10–12 M B) 7.1 1012 M C) 1.4 10–11 M D) 1.4 10 11 M 9. The pH of a 0.62 M KOH solution is _____. A) 0.21 B) 13.79 C) 1.21 D) 12.79 10. The hydrogen ion concentration of a solution having a pH of 2.42 is _____. A) 3.8 10 –3 M B) 3.8 10 –2 M C) 3.8 10 2 M D) 2.4 10 –3 M 11. Which of the following is true for a solution having pH < 7? A) [H+] < 10 –7 M B) [OH–] > 10 –7 M C) It is a basic solution. D) [H+] > 10 –7 M 12. Which of the following statements is true? A) pOH > 7; The solution is basic. B) pH > 7; The solution is acidic. C) pH< 7; The solution is acidic. D) pOH < 7; The solution is acidic. 13. The pOH of a solution is 9.40. Its hydrogen ion concentration is _____. A) 2.5 10 –3 M B) 2.5 10 –5 M C) 1.5 10 –5 M D) 2.5 10 –9 M 14. The number of moles of KOH in 5.50 mL of a 0.360 M KOH solution is _____. A) 1.98 10 –2 B) 1.52 10 –3 C) 1.98 10 –3 D) 1.98 Page 2 15. Which one of the following statements is true? A) The conjugate base of a strong acid is strong. B) The conjugate base of a strong acid is weak. C) The conjugate acid of a weak base is weak. D) H2SO4 is a strong acid. Therefore, HSO4– is a strong conjugate base. 16. Which one of the following statements is true for a 1.0 M solution of a weak acid HA? A) pH = 0 B) [H+] >> [A–] C) [H+] = [A–] D) pH < 1 17. Which of the following statements is true for a 1.0 M solution of a strong acid HA? A) [A–] > [H+] B) [HA] = 1.0 M C) pH = 1 D) pH = 0 18. Which of the following statements is true with respect to the reaction below? A) B) C) D) The reaction favors the formation of F–(aq). F– is a stronger base than OH–. Hydrofluoric acid is a weaker acid than water. The reaction favors the formation of HF(aq). 19. Which of the following statements is true with respect to the reaction below? A) B) C) D) The reaction favours the formation of CH3COO–. CH3COO– is a weaker base than Cl–. Cl– is a weaker base than CH3COO–. The reaction favours the formation of HCl. 20. Which of the following statements is correct with respect to an acid? A) Stronger acids have smaller Ka values. B) Weaker acids have smaller Ka values. C) The strength of an acid is inversely proportional to its Ka value. D) Weaker acids have greater H+ concentration. 21. The pH of a 0.1 M benzoic acid solution (Ka = 6.5 10–5) is _____. A) 3.6 B) 2.6 C) 2.0 D) 1.6 Page 3 22. What is the concentration of CH3COOH (Ka = 1.8 10–5) in 50.0 mL of a 0.0187 M CH3COOH solution? A) 0.0007 M B) 0.0181 M C) 0.0087 M D) 1.87 10–3 M 23. The pH of a 0.010 M weak monoprotic acid is 6.20. The Ka of the acid is _____. A) 4.0 10–11 B) 2.0 10–5 C) 4.0 10–8 D) 4.0 10–7 24. The percent ionization of 0.20 M benzoic acid (Ka = 6.5 10–5) is _____. A) 18 B) 1.8 C) 4.3 D) 43 25. A 0.040 M monoprotic acid solution is 14% ionized. Its Ka value is _____. A) 9.1 10–4 B) 4.6 10–3 C) 2.3 10–6 D) 9.2 10–5 26. The percent ionization of a 0.20 M acetylsalicylic acid solution (Ka = 3.0 10–4) is _____. (Acetylsalicylic acid is monoprotic) A) 39% B) 0.39% C) 3.9% D) 4.9% 27. The pH of a 0.30 M solution of a weak base is 10.66. What is the Kb of the base? A) 4.8 10–10 B) 1.85 10–5 C) 7.0 10–8 D) 7.0 10–7 28. The value of Kb for NH3 is 1.8 10–5. The value of Ka of NH4+ is _____. A) 5.6 10–10 B) 5.6 104 C) 1.8 10–19 D) 1.8 10–9 Page 4