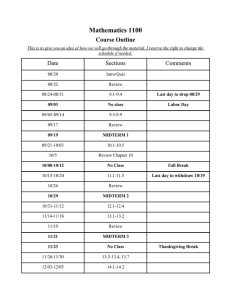
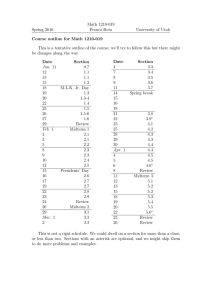
Sample Midterm Chem quiz 1 / 1 point Qualitatively predict the acidity/basicity of a 1.0 M solution of H3O+HSO41) Weakly Acidic 2) Strongly Basic 3) Neutral 4) Strongly Acidic 5) Weakly Basic 1 / 1 point Which indicator is best suited for the titration of 0.05 M C5H5N (pKb = 8.82) with 0.05 M HCl? 1) Phenol red (pKHIn = 7.60) 2) Bromophenol blue (pKHIn = 3.80) 3) Alizarin yellow-R (pKHIn = 11.15) 4) Methyl red (pKHIn = 5.30) 5) Thymolphthalein (pKHIn = 9.85) 1 / 1 point What is the pH of a solution that is 2.0 M CH 3COOH and 0.25 M CH3COONa? (CH3COOH ; Ka = 1.8 x 10-5) A) 3.84 1-1 Sample Midterm B) 5.34 C) 4.93 D) 4.21 E) 4.74 1 / 1 point 10.00 mL of a weak acid solution, HA (Ka = 2.63 × 10-7) is titrated with a strong base (0.100 M). If 3.98 mL of base gives a pH of 6.475, what is the concentration (in mol L-1) of the original weak acid solution? 1) 0.159 2) 0.0764 3) 0.193 4) 0.0905 5) 0.142 1 / 1 point In the reaction, 2A + B → 3C + 4D, the average rate of reaction is given by A) Δ[A]/(2Δt) B) (-2Δ[B])/(3Δt) C) Δ[C]/(3Δt) 1-2 Sample Midterm D) (-4Δ[D])/Δt E) (4Δ[D])/Δt 100 % 100 % Done 1-3