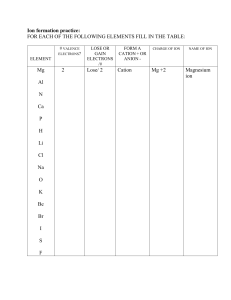
Ion formation practice: FOR EACH OF THE FOLLOWING ELEMENTS FILL IN THE TABLE: # VALENCE ELECTRONS? ELEMENT Mg Al N Ca P H Li Cl Na O K Be Br I S F 2 LOSE OR GAIN ELECTRONS /# FORM A CATION + OR ANION - Lose/ 2 Cation CHARGE OF ION Mg +2 NAME OF ION Magnesium ion Fill in the blanks: 1. Ions are formed when atoms ______ or _____electrons. 2. Atoms gain or lose electrons to become ___________. 3. Atoms follow the __________ rule which says that an atom wants a completely ____ outer shell or a completely _______ outer shell to be stable. 4. The octet rule states that: 5. _____ are positive ions formed by a _______ losing electrons. 6_______ are negative ions formed by a ___________ gaining electrons. Define the following words: Valence electrons Ionic bond Covalent bond Metal Nonmetal Metalloid Cation Anion Ionic charge Compound Chemical bond Molecular bond Polyatomic ions