Name____________________________________________________________
Chem Ch 4.1 -.2 Concepts Review
Per_____ Date______________________
Unit: Atomic Structure - Electrons and Electron Configurations
Standard: I can describe the nature of electron arrangement within atoms (nature/behavior of electrons, electron configurations)
Prof.Scale 1: Describe the contributions of Bohr and Schrodinger to the understanding of electrons.
Ch 4.1 Concepts
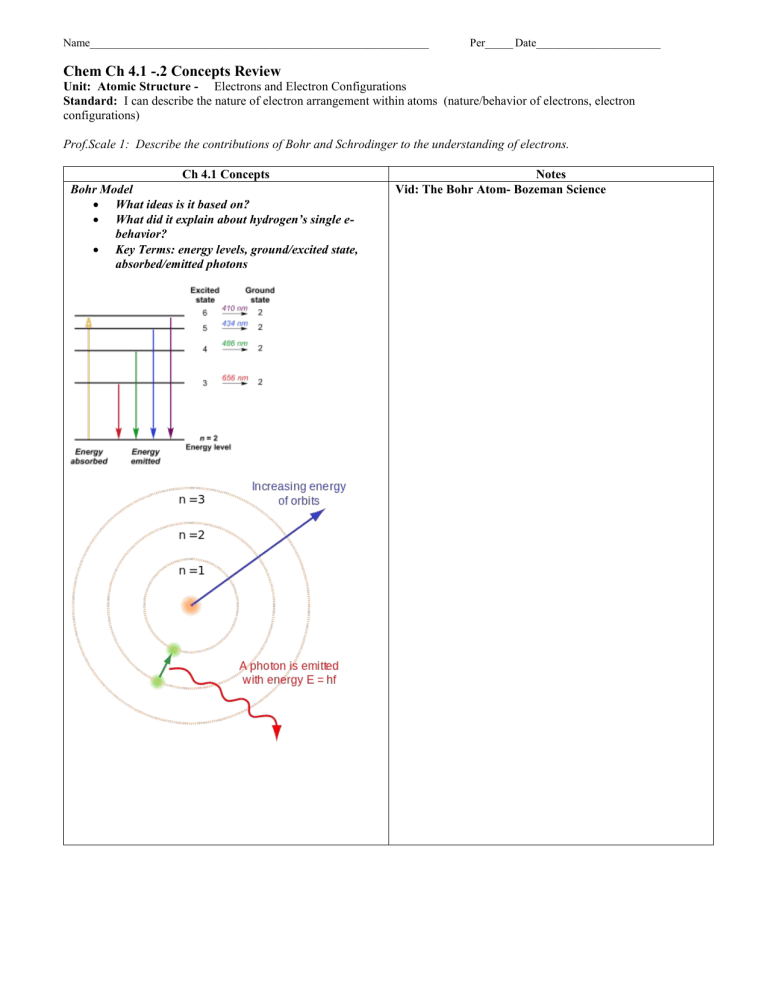
Bohr Model
What ideas is it based on?
What did it explain about hydrogen’s single e- behavior?
Key Terms: energy levels, ground/excited state, absorbed/emitted photons
Notes
Vid: The Bohr Atom- Bozeman Science
Ch 4.2 The Quantum Model of the Atom
Concepts
De Broglie Model
How did the model look at how electrons behave?
Notes
Vid: de Broglie Waves - Sixty Symbols
Ch 4.2 The Quantum Model of the Atom
Concepts
Heisenberg Uncertainty Principle
From the text, p 105 what was the problem about the wave nature of an electron?
Notes
Vid: What is the Heisenberg Uncertainty Principle?
Vid: The Heisenberg Uncertainty Principle Explained






