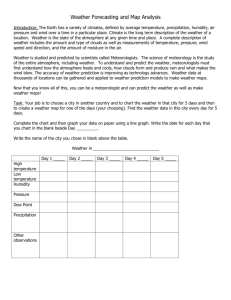
Name: ____________________________________________ Date: _____________________ Humidity Lab Materials List Each group needs: ● ● ● ● 1 dry sponge 1 plastic spoon water, 1 cup paper and pencil, to keep a tally Introduction Under normal Earth conditions, water exists in any of three physical states: solid (ice, snow), liquid (which we typically call water) or gas (humidity, water vapor [steam]). According to meteorologists, humidity can be absolute or relative, giving us the terms absolute humidity and relative humidity. In common usage, the adjective is often dropped, and the speaker (or writer) just says "humidity." Relative humidity is usually what the media announcers mean when they say "humidity," and it is useful in determining conditions for human comfort. But, it can be confusing because its value varies with air temperature. For example, the morning may have a relative humidity of 78%, which by afternoon drops to 53% as the air temperature rises. Understanding the concept of saturation is important to understanding relative humidity. Saturation is defined as the condition in which air at a specific temperature contains all the water vapor it can hold; 100% relative humidity. At saturation, the partial pressure of water vapor in the atmosphere is at its maximum level for the existing ambient temperature and pressure. At saturation, equilibrium exists between water vapor and liquid water, and there is no net evaporation or condensation. Above saturation, some of the water in the air condenses to form droplets or clouds (collections of droplets). By better understanding humidity, one can better understand local weather. Given the temperature of a volume of air and its pressure, we can determine a saturation value. We can saturate a parcel of air by adding more water vapor to it (through evaporation or mixing with another parcel of more humid air), or by cooling the parcel down to its saturation temperature. Both processes are at work continually in the atmosphere, but the latter is more familiar to us as it forms fog or dew (or frost if cold enough). The saturation temperature of the ambient air is commonly called the dew point temperature or simply the dew point (or frost point if it is below freezing). You might hear a weathercaster or meteorologist discuss the dew point of a particular air mass. Dew point varies little within an air mass. When the ambient air temperature equals the dew point, the relative humidity is 100% and the air is saturated. If the air temperature falls lower, water vapor begins to condense into very small liquid droplets to form clouds or fog (if it is near the ground). If the surface temperature of an object (vegetation, rooftop or car exterior) falls below the dew point while most of the surrounding air remains above it, dew forms through the condensation of water vapor onto that surface (or ice crystals, we call it frost, if it is below freezing). Pre-Assessment Activity Questions: What is relative/humidity? How does it affect the weather? 2. What variable is humidity dependent on? 3. At saturation, equilibrium exists between what to phase of water? 1. 4. What happens when the atmosphere reaches above saturation? 5. How is dew or fog formed? What phase change allows this to occur? 6. What does it mean when the air is 100% saturated? How much water is in the sponge initially? 8. Define the following: a. absolute humidity: b. dew point: c. humidity: d. meteorologist: e. partial pressure: f. relative humidity: g. saturation: 9. How are clouds formed? 7. Procedure: Squeeze the sponge to verify that they contain no water. 2. One spoonful at a time, slowly and carefully pour water onto the sponge. 3. Count how many spoonfuls of water are being added; it helps to have one group member keep a tally. 1. Figure 2. Sponge and water model activity, before and after. 4. After a few spoonfuls, Answer the following: a. What is happening to the water? b. Where is it going? c. What do you think is going to happen as you keep filling the sponge with water? d. Can you put water into this sponge forever? e. Will we be counting forever? Resume adding water to the sponge by the spoonful until the sponge starts to drip water (reaches saturation). 6. How many spoonfuls of water did it take for your sponge to be 100% saturated? (This means it cannot hold any more water and it is ready to drip.) ___spoonfuls 7. Use the following calculation to determine the percent saturation for the sponge at each stage (number of spoonfuls) of adding water. 5. # of spoonfuls ÷ total # of spoonfuls for saturation X 100% = percent saturation (%) Example calculation: If the sponge was saturated with 15 spoonfuls of water, for 9 spoonfuls, the calculation is 9 ÷ 15 X 100% = 60% saturation. 8. Record your data and calculations in the table below. Data: # Spoonfuls % Saturation Post Assessment Activity: # Spoonfuls % Saturation 1. Explain what has happened to the sponge. Why is water dripping from it? 2. What does the sponge represent? 3. How does the water dripping from the sponge act like rain or a cloud? 4. What is another name that means something is full (of water, like the air or the sponge). sponge full (100% saturated) = ___ spoonfuls of water 5. How many spoonfuls did it take to reach 100% saturation of your spong? Is there a difference among the groups data? 6. How many tablespoons of water had been poured into the sponge when it was halfway full of water? i. sponge half full (50% saturated) = ___ spoonfuls of water 7. How many spoonfuls of water were in the sponge when the sponge was empty? i. sponge empty (0% saturated) = _____ spoonfuls of water 8. Using your data answer the following questions: ● How many tablespoons of water would be in the sponge if the sponge were nearly ready to drip, but not dripping yet? ● How many tablespoons of water would be in the sponge if it were nearly empty, but not totally empty? ● How many spoonfuls are required to reach 40% saturation? ● What would happen if you added more spoonfuls than are required to reach 100% saturation? etc.) Extension: Meteorologists can measure how close is it to raining by using a humidity scale for water present in the air. Use the Relative Humidity Graph link to answer the following questions:(Note that the graph handout is much more complex and is dependent on temperature.) 1. At 72 degrees F and 0.010 lbs of water what is the relative humidity? 2. How many pounds of water are in the atmosphere when it is 90 degrees F and 20% relative humidity? 3. What is the temperature when it is 0.012 lbs of water in the atmosphere and 90% relative humidity? 4. At 85 degrees F and 0.0008 lbs of water what is the relative humidity? 5. How many pounds of water are in the atmosphere when it is 101 degrees F and 20% relative humidity? 6. What is the temperature when it is 0.009 lbs of water in the atmosphere and 40% relative humidity?