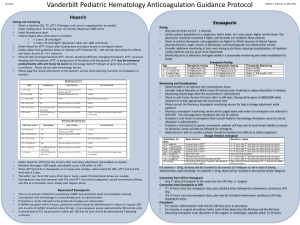
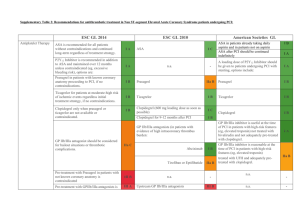
STEEPLE Trial Safety and Efficacy of Intravenous Enoxaparin in Elective Percutaneous Coronary Intervention: an International Randomized Evaluation (STEEPLE) Presented at The European Society of Cardiology Hot Line Session 2005 Presented by Dr. Gilles Montalescot STEEPLE Trial 3528 patients age >18 years undergoing non-emergent single or multi-vessel PCI (performed with a femoral approach) Randomized 25% female, mean age 64 years, mean follow-up 30 days GP IIb/IIIa inhibitors were used in 41% of patients, and aspirin in 85% Drug-eluting stents were used in 57% of patients and multivessel PCI was performed in 16% of patients IV enoxaparin IV enoxaparin 0.5 mg/kg 0.75 mg/kg n=1070 n=1228 Activated clotting time (ACT) – adjusted IV unfractionated heparin (UFH) regimen With GP IIb/IIIa (50-70 IU dose): target ACT 200-300 Without GP IIb/IIIa (70-100 IU dose): target ACT 300-350 n=1230 Primary Endpoint: Non-CABG related major and minor bleeding by 48 hrs post-PCI Secondary Endpoint: Percent of patients reaching target anticoagulation levels at the start and end of the procedure; composite of non-CABG major bleed through 48 hrs; allcause mortality; myocardial infarction; urgent target vessel revascularization at 30 days www. Clinical trial results.org Presented at ESC 2005 STEEPLE Trial: Primary Endpoint at 48 hours Analysis of non-CABG major or minor bleeding (%) 10% p=0.014 vs UFH 6.0% p=0.052 vs UFH • The primary endpoint of non-CABG major or minor bleeding was lower in those groups treated with enoxaparin 8.7% 6.6% 5% 0% Enoxaparin 0.5 mg/kg Enoxaparin 0.75 mg/kg www. Clinical trial results.org UFH • The lower bleeding rate associated with enoxaparin was observed both in the subgroup of patients intended to be treated with GP IIb/IIIa inhibitors, as well as in a per protocol analysis Presented at ESC 2005 STEEPLE Trial: Primary Endpoint at 48 hours Analysis of major bleeding (%) 2.8% 3% 2% p=0.005 vs UFH 1.2% p=0.007 vs UFH 1.2% 1% • Major bleeding occurred in 1.2% of each of the enoxaparin groups and 2.8% in the UFH group • The primary endpoint of major bleeding was 57% lower in the enoxaparin groups compared with the UFH group 0% Enoxaparin 0.5 mg/kg Enoxaparin 0.75 mg/kg www. Clinical trial results.org UFH Presented at ESC 2005 STEEPLE Trial: Primary Endpoint at 48 hours Analysis of minor bleeding (%) 6% P=0.315 vs UFH P=0.530 vs UFH 5.4% 5.9% 4.9% • Minor bleeding occurred in 4.9% (0.5 mg/kg) and 5.4% (0.75 mg/kg) in each of the two enoxaparin groups and 5.9% in the UFH group 4% 2% 0% Enoxaparin 0.5 mg/kg Enoxaparin 0.75 mg/kg www. Clinical trial results.org UFH Presented at ESC 2005 STEEPLE Trial: Secondary Endpoint Analysis of patients reaching target anticoagulation levels at the start and end of procedure (%) 100% 80% p<0.001 vs UFH p<0.001 vs UFH 91.7% • The percent of patients reaching target anticoagulation levels at the start and end of the procedure was significantly lower among the UFH treatment group compared with the two enoxaparin treatment groups (78.8%, 91.7% vs 19.7%) 78.8% 60% 40% 19.7% 20% 0% Enoxaparin 0.5 mg/kg Enoxaparin 0.75 mg/kg www. Clinical trial results.org UFH Presented at ESC 2005 STEEPLE Trial: Secondary Endpoint Composite endpoint of non-CABG major bleed through 48 hours, all-cause mortality, MI, or urgent target vessel revascularization at 30 days (%) p=NS 10% 7.2% 7.9% • The composite secondary endpoint was numerically lower among the two enoxaparin treatment groups compared with the UFH treatment group (7.2%, 7.9% vs 8.4%) 8.4% 5% • There was no difference in death or MI individually 0% Enoxaparin 0.5 mg/kg Enoxaparin 0.75 mg/kg www. Clinical trial results.org UFH Presented at ESC 2005 STEEPLE Trial Summary • Among patients undergoing non-emergent PCI, treatment with reduced dose enoxaparin was associated with lower rates of major or minor bleeding by 48 hours post-PCI compared with treatment with ACT-driven UFH. • Patient enrollment in the enoxaparin 0.5 mg/kg treatment group was discontinued by the data safety monitoring committee near the end of the trial at the objection of the steering committee, due to a difference in mortality between the three groups (p=0.02). • With full 30 day data, neither mortality, MI, nor urgent target vessel revascularization differed between the three groups. • Further investigation is still necessary, but the results from this trial show that the use of enoxaparin at lower doses in the catheterization laboratory may offer a potential safety advantage with lower bleeding events relative to ACT guided UFH. www. Clinical trial results.org Presented at ESC 2005