Uploaded by
Saba Mirbaha _ Student - WakeForestHS
Unit 6 Chemistry Review Guide: Solutions, Acids, Bases
advertisement

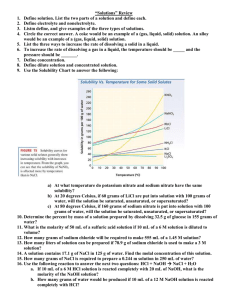
Unit 6 Review Guide 1 SOLUTIONS 1.) If you dissolve 50g of ammonium chloride in 100g of water at 50°c, what type of solution (saturated, unsaturated, or supersaturated) would you make? 2.) How many grams of sodium sulfate can be dissolved in 50g of water at 31°c? 3.) At what temperature can you dissolve 85g of potassium nitrate in 100g of water? 4.) How many grams of potassium chlorate can be dissolved in 250g of water at 75°c? 5.) If you dissolve 100g of sodium sulfate in 150g of water at 20°c, what type of solution (saturated, unsaturated, or supersaturated) would you make? Answer the following questions on a separate sheet of paper. Use the formulas on your yellow objective sheet. 6.) Calculate the molarity of 29.25 grams of NaCl in 2.0 liters of solution. 7.) Calculate the molarity of 34.2 grams of sugar, C12H22O11 in 500. mL of solution. 8.) If I dilute 250 mL of 0.10 M lithium acetate solution to a volume of 750 mL, what will the concentration of this solution be? 9.) Calculate the molarity of 0.060 moles NaHCO3 in 1500. mL of solution. 10.) Calculate the mass of NaOH contained in 250. mL of a 0.05M solution. 11.) What volume would you need to dissolve 250g of calcium chloride to make a 0.50M solution? 12.) If you have 340 mL of a 0.5 M NaBr solution, what will the concentration be if you add 560 mL more water to it? 13.) If you leave 750 mL of 0.50 M sodium chloride solution uncovered on a windowsill and 150 mL of the solvent evaporates, what will the new concentration of the sodium chloride solution be? 14.) To what volume would you need to add water to the evaporated solution in problem 13 to get a solution with a concentration of 0.25 M? 2 ACIDS AND BASES Use the formulas on your yellow objective sheet to help you fill out the following chart. Round all answers to 3 sig figs. pH [ H3O+ ] pOH [ OH– ] ACID or BASE? 3.78 3.89 x 10–4 M 5.19 4.88 x 10–6 M 8.46 8.45 x 10–13 M 2.14 2.31 x 10–11 M If it takes 25 mL of 0.05 M HCl to neutralize 345 mL of NaOH solution, what is the concentration of the NaOH solution? If it takes 50 mL of 0.5 M potassium hydroxide solution to completely neutralize 125 mL of sulfuric acid solution, what is the concentration of the sulfuric acid solution?