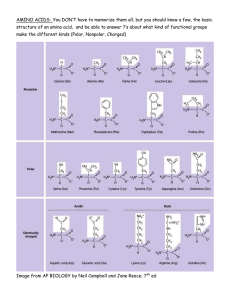
AMINO ACIDS • Amino acids are organic compounds containing an amine group, a carboxylic acid group and a side chain that varies between different amino acids • Amino Acids are the building blocks of proteins, important in many biological molecules, such as enzymes , co- enzymes, precursors for the biosynthesis of molecules, and have many functions in metabolism • An amino acid molecule react with other and made linkage to form polymer of amino acids called proteins. This condensation reaction yields the newly formed peptide bond and a molecule of water. AMINO ACID ANALYSIS Amino acid analysis is a technique used to determine the amino acid composition or content of proteins. The sequence of the amino acids in a protein or peptide determines the properties of the molecule. • It is used to quantify protein and peptides. • To determine the identity of proteins or peptides. • To detect atypical amino acids present in a protein. • Amino acid analysis is also essential in the diagnostic of inherited metabolic disorders such as phenylketonuria (PKU) and maple syrup urine disease (MSUD) and different other diseases TYPES OF AMINO ACID ANALYSIS • The following 3 groups of tests are important • Screening tests • Quantitative tests to monitor treatment or confirm an initial diagnosis • Specific tests that identify an unknown amino acid or metabolite • The Amino Acid Quantitative tests Analyzer perform the • The Amino Acid analyzer analysis all known amino acids beside it also analyses the following Taurine Urea Asparagine Citrulline Ornithine Homocysteine α-Aminoadipicacid α-Aminobytyricacid Hydroxyproline 1-methyl histidine Ammonia It is necessary to hydrolyze a protein/peptide to its individual amino acid constituents before amino acid analysis. Methods used for amino acid analysis are usually based on a chromatographic separation of the amino acids present in the test sample. An amino acid analysis instrument is generally a lowpressure or high-pressure liquid chromatography or GC. A detector is usually present as an ultraviolet-visible or fluorescence detector . • A recording device (e.g., integrator) is used for transforming the analog signal from the detector and for quantitation. SAMPLE PREPARATION • Accurate results from amino acid analysis require purified protein and peptide samples. • Buffer components (e.g., salts, urea, detergents) can interfere with the analysis so removed from the sample before analysis 1. Precipitating the protein from the buffer using an organic solvent (e.g., acetone) and drying in a vacuum centrifuge 2. Acid precipitation (by using Sulfosalisilic acid) 3. Dialysis against a volatile buffer or water 4. Ultrafiltration for buffer replacement with a volatile buffer or water 5. Gel filtration. pH ADJUSTMENT • Each amino acid has isoelectric pH (PI) and its charge will be natural at this pH • By increasing H ions (decreasing pH) it will be positively charged and visa versa by increasing OH ions (increasing pH) it will be negatively charged • Therefore, when preparing the sample the pH should be 1.8-2 because this pH is less than the PI of all amino acids so that all amino acids will positively charged PROTEIN HYDROLYSIS Acid hydrolysis is the most common method for hydrolyzing a protein sample before amino acid analysis. However, some of the amino acids can be destroyed A time-course study (i.e., amino acid analysis at acid hydrolysis times of 24, 48, and 72 hours) is often employed to analyze the starting concentration of amino acids that are partially destroyed or slow to cleave METHOD FOR HYDROLYSIS Hydrolysis Solution: 6 N hydrochloric acid containing 0.1% to 1.0% of phenol. ( phenol is to prevent halogenation of tyrosine) Liquid Phase Hydrolysis a. Place the protein sample in a hydrolysis tube, and dry. b. Add 200 μL of Hydrolysis Solution per 500 μg of protein. c. Freeze the sample tube in a dry ice-acetone bath, and flame seal in vacuum. d. Samples are typically hydrolyzed at 110ºC for 24 hours in vacuum or inert atmosphere to prevent oxidation. Longer hydrolysis times (e.g., 48 and 72 hours) are investigated if there is a concern that the protein is not completely hydrolyzed. DERIVATIZATION • Typically, HPLC is used for the analysis of amino acids. • However, GC also be used and availability of instrument & operation costs can make it a better choice. • The polar nature of amino acids requires derivatization prior to GC analysis. • The derivatization make an analyte more volatile, less reactive, and improve its chromatographic behavior. • Derivatization replaces active hydrogens, OH, NH2, and SH polar functional groups of AA with a nonpolar moiety. • Silylation is a very common derivatization technique & useful for a wide variety of compounds. • The main disadvantage of this method is its sensitivity to moisture. The presence of moisture results in poor reaction yield and instability of the derivatized analytes. • Other eg are halogination, methylation, glycosylation etc. SAMPLE ANALYSIS • • • • • The sample go through 4 steps Autosampler Separation column reaction Coil reaction Photometer AUTOSAMPLER • Before samples are analyzed the commercially available standard solution have to be analyzed to calibrate the instrument • The amino acid glutamine is not present in the standard since it decomposes quickly. Therefore a fresh solution of glutamine is prepared (1.0 µmol / ml ) and injected then the freshly prepared sample is injected and glutamine peak is identified from retention time • Following sample preparation the sample is inserted in the specified place then the autosampler inject 130 µl of the sample and pass it to separation column SEPARATION COLUMN REACTION • In this situation the separation column is the stationary phase and the buffers are the mobile phase. • A special pump pumps the buffer to the separation column. • In the separation column ion exchange reaction take place and the positively charged amino acids are bound to the negatively charged sites in the separation column • The separation depends on different factors for example ion size, Adsorption METHOD FOR ANALYSIS Ion-exchange chromatography with postcolumn ninhydrin detection is one of the most common methods employed for quantitative amino acid analysis. a Li-based cation-exchange system is employed for the analysis of the complex physiological samples, and the faster Na-based cation-exchange system is used for the simple amino acid mixtures • Separation of the amino acids on an ion-exchange column is accomplished through a combination of changes in pH and cation strength. • A temperature gradient is often employed to enhance separation. COIL REACTION • When the amino acid reacts at 130oC with ninhydrin, the reactant has characteristic purple or yellow color. • Amino acids, except imino acid, give a purple color, and show the maximum absorption at 570 nm. • The imino acids such as proline give a yellow color, and show the maximum absorption at 440 nm • The postcolumn reaction between ninhydrin and amino acid eluted from column is monitored at 440 and 570 nm. • and the chromatogram obtained is used for the determination of amino acid composition. PHOTOMETER • After the ninhydrin reaction, The resultant colored species are detected with a spectrophotometer where the absorption of the colored complex is measured at two wavelength length 570 and 440 nm • The quantity of the colored complex produced is directly proportional to the concentration of the particular amino acid present in the sample RECORDER • Photometer is linked to a two channel recorder where a series of peaks representing the amino acids are recorded • The amino acids are identified by the comparison of the retention times of the components in the specimen with reference compounds • The information is transferred to a specific computer program where Quantitation of each amino acid could be done by comparison of specimen peak area or height with standards. proline Hydroxyproline