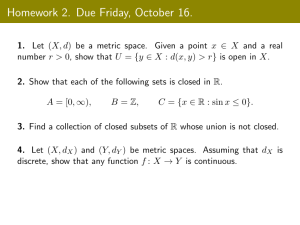
• Please Don’t forget to return Exercise 1 by the printer
Metrics and Common
Computations
Page 7
Metrics
• We use the English or British-American system of measurements but professional world is converting to the Metric system of measurement.
Length:
• English (Inch, foot, yard, mile)
• Metric (Millimeter, centimeter, Meter, Kilometer)
Volume:
• English (Pint, Quart, gallon)
• Metric (Milliliter, deciliter, Liter, Kiloliter)
Mass:
• English (Ounce, pound, ton)
• Metric (Milligram, gram, Kilogram, metric ton)
Temperature:
• English (Degree Fahrenheit)
• Metric (Degree Celsius or Centigrade)
The difference between metric system is based on decimal system and units of 10.
• Convert to a larger unit: DIVIDE numbers by 10 if you are getting bigger (same as moving decimal point one space to the left).
• Convert to smaller unit: MULTIPLY numbers by 10 if you are getting smaller (same as moving decimal point one space to the right).
Metric Units
1. Length: Meter. 1m = 39.37 inch or 1.094 yards.
2. Mass: (g, kg). 1g = 0.035 Ounces.
• Mass is not weight.
• Mass: is the quantity of matter in the object.
• Weight: force exerted by object as a result of gravity.
• When we determine the weight of an object we are measuring objects mass and its associated gravity pull.
3. Volume: L (liter). 1L = 1.057 quarts.
• Another measure of volume is based on linear measurement. When any linear measurement is cubed, it becomes a volume.
• For example: 1 meter length (x) 1 meter width (x) 1 meter height = 1 meter cubed or (1 cubic meter).
4. Temperature: Celsius
• Freezing water point = 32 F or 0 C.
• Boiling water Point = 212 F or 100 C.
• F & C conversion Formula:
Temp C = T F – 32
1.8
Temp F = (T C x 1.8) + 32
Metric Conversion
• 1 st Question ask your self ( I am converting to large or smaller unit?)
• 2 methods: moving Decimal Points or Dimensional analysis.
Scientific Notation
• Simplify the Expression of the number. (done by a power of 10).
Look At Table page 10
Look At method 1,2 Examples page 11
Look At Metric Conversion sheet.
Answer Question page 12
Look at Scientific Examples page 12
Answer questions page 12
Proportions
Equivalency between 2 ratios
Proportions……..
Solve Questions Page 13
Mixtures
Any two substances mixed together
Draw….
1. If solvent water >>> Aqueous Solution.
2. Suspension >>> Particles are insoluble “it will not dissolve” >>> settle to the bottom.
3. Colloid Suspension >>> Colloids are very small particles that do not dissolve but are small enough to stay in suspension without periodic mixing. E.g. Plasma Proteins.
4. Homogenous Solution: solutes are evenly dispersed throughout the solvent.
5. Heterogeneous Solution: solutes are not evenly dispersed throughout the solvent.
6. Saturated Solution: has Maximum solute particles so if we add more >>> Precipitate to the bottom.
Types Of Solutions
1. Percent Solution
2. Molarity
3. Osmolarity
• Percent Solution: the number of parts of solute per 100 parts of solution.
• Q: physiologic saline >>> 0.87% NaCl or 0.87 g NaCl mixed in enough water to make 100 mL of physiologic saline solution.
• Given the following % solutions, determine the amount of solute and the approximate amount of solvent in each:
1. 1 L of physiologic saline solution?
1 L = 1000 mL >>> 1000 mL / 100 mL = 10.
So 10 x 0.87 = 8.7 g in 1000 mL.
Solute = 0.87 g
Solvent = 100 ml
2. 100 mL of 10% glucose solution?
10 g glucose and enough water to make 100 mL of the solution.
• Molarity: number of moles of solute dissolved in 1 L of solution
(moles/L).
Steps:
• Check atomic weight. “g”
• How many L asking for?
• Calculate.
Q: Determine the amount of solute in 1 L of the following solutions?
(Na=23 g - Cl = 35.5 g)
# 4 molar solution of NaCl ? (23 +35.5) x 4 = 234
Q: How many grams of the solute would be required to make each of the following solutions?
2 L of 4 molar solutions of NaCl ? 2 x 234 g = 468
Solve Question Page 14
• Osmolarity: Number of particles in solution.
• One molecule of dry undissolved NaCl = 1 particles “osmolarity is 1”
• one molecule of NaCl dissolved in water “dissociates” = 2 osmolarity
• Osmolarity: we count each ion as a separate particle.
Open page 15, look at the picture and solve questions.
Means ….
Open Page 16 and complete the Table
Graphs
Shows the relationship between two sets of values or variables
(dependent + independent).
Dependent: variable that you are monitoring in an experiment.
Independent: variable that you manipulate “change” during experiment.
• If both Axis Y and X Increased or decreased >>> direct relationship.
• if both Axis y and X went in opposite direction >>> Indirect relationship.
• Linear relationship >>> Straight Line.
• Curvilinear >>> Curved Line.
Draw…