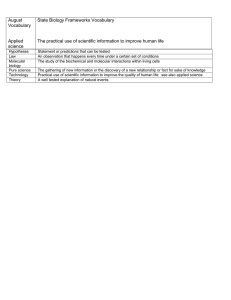
Forces and Properties Questions to Answer Directions: Answer one of the questions below in your own words, using the principles of CER. You should be using knowledge of inter- and intra- molecular forces in conjunction with another concept from this unit. When possible expand the given question to include other familiar examples of the same situation (aka, take the concept further or turn it around and go the other way). If a diagram or graph is appropriate, use it. Does a mathematical relationship (aka formula) help explain it, use it. For maximum points, an illustration at the molecular level should be used. You will need to submit a presentation to the communal shared folder that includes citations and a link to at least one video that supports, or further elaborates on your answer. Finally, you will present your answer to the class in a 2-4 min presentation- be clear, succinct, and thorough. Questions: 1. Why is water a liquid at room temperature and ammonia is a gas? Why is methane a gas and octane a liquid at room temperature? 2. Why does ice float on top of water but other solids sink in their liquid? 3. Why are some ionic compounds more soluble in water than others? 4. If all noble gases are basically ideal gases, why do they have different boiling points? 5. What’s the story behind the saying: “Slower than molasses on a cold winter’s day”? 6. Why does water from more circular droplets on a countertop than rubbing alcohol? 7. Why do alcohol and water mix, but we can still have layered “mixed” drinks? 8. Why does a water meniscus curve the way it does? Why is mercury’s meniscus the other way? 9. Why does a steam burn hurt so much more than a hot water burn? Why does touching dry ice cause a “burn”? 10. Why does water boil faster at a higher elevation, but the food takes longer to cook? 11. How do you get carbon dioxide to exist in all three phases at the same time? 12. Why does ice melt under the blade of an ice skater? 13. Why will an empty, closed, plastic water bottle collapse in upon itself as you come down the mountain? 14. Why does a helium balloon change size as you go in and out of the cold casino on a hot summer day? 15. Why does the pressure gauge on your car’s tires say “low” in the winter and you know no gas has escaped? 16. Why does it take time for a balloon to naturally deflate, but you can smell perfume/cologne across the room almost instantaneously? 17. Why would a scuba diver need to surface well before the tank becomes empty? 18. Why do we rarely see dew on the ground in Las Vegas? 19. What are the optimal conditions to make carbonated beverages? Not technically covered by AP (colligative properties), but still really good questions that you should be able to answer… 20. Why do firefighters create a dike around a tanker fuel spill? 21. Why does adding salt to water really only flavor the food, rather than raise the boiling temperature? 22. Why do they spread calcium chloride rather than sodium chloride on icy roads? 23. Why do our fingers “prune” when we go swimming? Presenter's Name: _________________________________ Question # ______: Time (2-4min): _________ Concepts covered to answer question: CLaim: A single sentence answering the question. EVidence: at least 3 pieces of content to help explain the claim, referring us to specific data, diagrams, charts, scientific concepts, calculations, graphs, etc. Reasoning: explain how the evidence explains the claim with logical connections and an explanation at the molecular level when possible. Use of linking phrases needs to be well done and will allow for a one time read through. SCORE= _____/25 0 1 2-3 4 5 CLAIM Does not make claim Makes inaccurate claim Incomplete claim Accurate claimanswers the question, mostly Accurate claim- clearly answers the question. EVIDENCE No relevant evidence provided Inappropriate evidence to support claim appropriately 1-2 pieces of evidence. Weak support of claim. 2 pieces of evidence. Support claim moderately well. 3 or more great qualitative and quantitative pieces of evidence that all support of claim well. REASONING No reasoning provided Reasoning does not justify the claim and/or is not a sound scientific explanation Connects some evidence to claim, but is not clearly explained. Difficult to understand without Q&A Clearly uses evidence to justify claim using good scientific principles, but does not connect all the parts together. Clearly & logically uses evidence to justify claim using good scientific principles and needs no clarifying questions. MOLECULAR LEVEL Missing molecular level May have words/pictures, but use is poorly done. Uses only words to explain what happens at the molecular level. Has a picture but explains it poorly OR explains well but has a poor picture. Explains with words AND pictures what happens at the molecular level. Additional Items (HW grade)= ______/ 15 _____/ 5pts- uploaded to correct folder before any presentations start _____/ 5pts- proper APA style citations _____/ 5pts- video link clearly marked and offers appropriate support to presentation for students that need more.