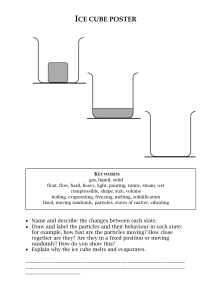
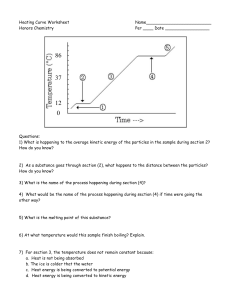
Year 10 Changes of State A test tube of crushed ice is taken out of a freezer and left in a warm room. The graph shows how the temperature in the test tube changes. 30 stage D 20 temperature in ºC 10 stage C 0 stage B stage A -10 (a) 0 5 10 15 20 25 30 35 40 45 50 55 60 time in minutes . What is happening to the ice at stage B? ..................................................................................................... (b) Describe the graph fully from start to finish, state what is happening at each lettered stage and explain the behavior of the particles. Be specific, quoting times and temperatures where possible. ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………….(6 marks) c) Suggest what might happen if heat were applied to the beaker at stage D. Why does this happen? …………………………………………………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………. (3 marks) Year 7 Particle theory A test tube of crushed ice is taken out of a freezer and left in a warm room. The graph shows how the temperature in the test tube changes. 30 stage D 20 temperature in ºC 10 stage C 0 stage B stage A -10 (a) 0 5 10 15 20 25 30 35 40 45 50 55 60 time in minutes . What is happening to the ice at stage B? ..................................................................................................... (b) Describe the graph fully from start to finish, state what is happening at each lettered stage and explain the behavior of the particles. Be specific, quoting times and temperatures where possible. ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………….(6 marks) c) Suggest what might happen if heat were applied to the beaker at stage D. Why does this happen? …………………………………………………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………. (3 marks) 7G Higher- Mark scheme (a) Melting (b) Level 4- identified state at stages A & C Level 5- Identified state at all stages. And explained motion of particles in a so;lid liquid & gas. Level6- explain what is happening during change of state and quoted time/temperature Level 7- Explained energy transfers/changes to explain stage B & D and why temperature doesn’t change. (c) Level 4- the temperature would increase. Level 5- Heat energy transferred to particles.