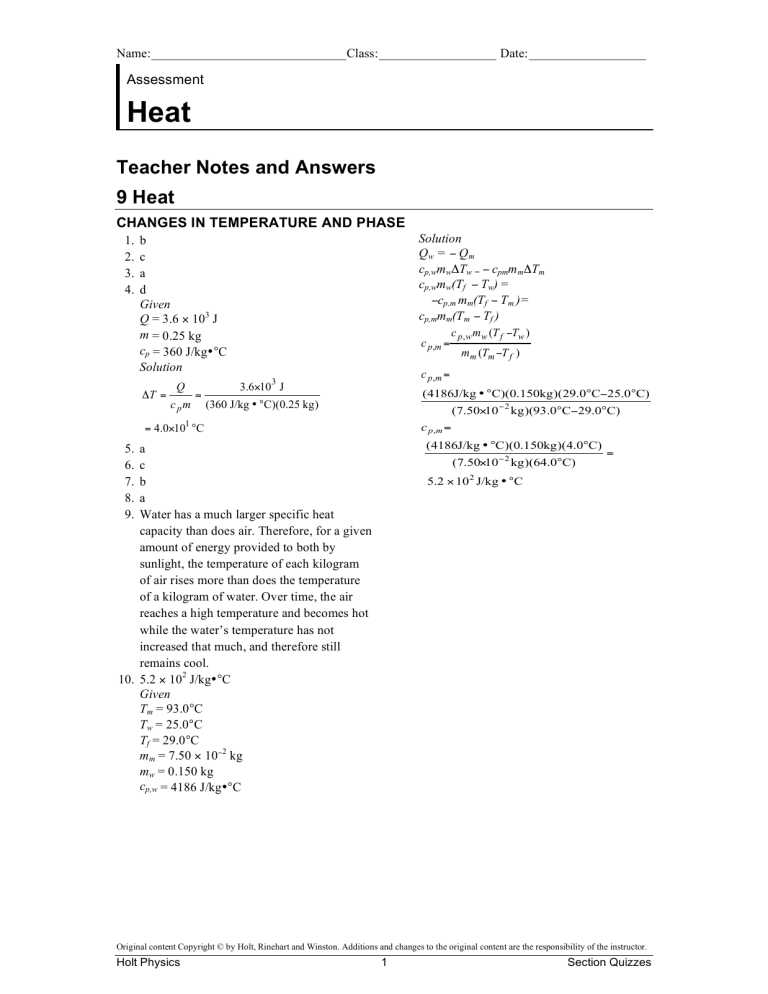
Name:______________________________Class:__________________ Date:__________________ Assessment Heat Teacher Notes and Answers 9 Heat CHANGES IN TEMPERATURE AND PHASE 1. 2. 3. 4. Solution Qw = Q m cp,wmwTw = cpmm mTm cp,wmw(Tf Tw) = cp,m mm(Tf Tm )= cp,mmm(Tm Tf ) b c a d Given Q = 3.6 103 J m = 0.25 kg cp = 360 J/kg•°C Solution T = c p ,m = c pm = mm (Tm T f ) c p,m = 3 Q c p , w m w (T f Tw ) 3.610 J ( 4186J/kg • °C)(0.150kg )(29.0°C25.0°C) (360 J/kg • °C)(0.25 kg ) (7.5010 2 kg )(93.0°C29.0°C) 1 c p ,m = = 4.010 °C ( 4186J/kg • °C)(0.150kg )(4.0°C) 5. 6. 7. 8. 9. a c b a Water has a much larger specific heat capacity than does air. Therefore, for a given amount of energy provided to both by sunlight, the temperature of each kilogram of air rises more than does the temperature of a kilogram of water. Over time, the air reaches a high temperature and becomes hot while the water’s temperature has not increased that much, and therefore still remains cool. 10. 5.2 102 J/kg•°C Given Tm = 93.0°C Tw = 25.0°C Tf = 29.0°C mm = 7.50 102 kg mw = 0.150 kg cp,w = 4186 J/kg•°C (7.5010 2 kg )(64.0°C) = 5.2 10 2 J/kg • °C Original content Copyright © by Holt, Rinehart and Winston. Additions and changes to the original content are the responsibility of the instructor. Holt Physics 1 Section Quizzes Name:______________________________Class:__________________ Date:__________________ Assessment Heat Section Quiz: Changes in Temperature and Phase Write the letter of the correct answer in the space provided. _____ 1. What is the quantity of energy needed to raise the temperature of a unit mass of a substance by 1°C called? a. latent heat b. specific heat capacity c. internal energy d. thermal energy _____ 2. Which property of a substance is not needed to determine the amount of energy transferred as heat to or from the substance? a. temperature change b. specific heat capacity c. volume d. mass _____ 3. The specific heat capacity of a substance is determined using a calorimeter containing water. Besides the substance’s mass and the change in temperature of the test substance, what other quantities must be measured in calorimetry? a. the mass, specific heat capacity, and temperature change of the water b. the volume, specific heat capacity, and temperature change of the water c. the density, specific heat capacity, and temperature change of the water d. the mass, thermal conductivity, and temperature change of the water _____ 4. A metal bolt in a calorimeter gives up 3.6 103 J of energy as heat to the surrounding water. The bolt has a mass of 0.25 kg and a specific heat capacity of 360 J/kg•°C. What is the change in the bolt’s temperature? a. 0.40°C b. 2.5°C c. 4.0°C d. 4.0 101 °C Original content Copyright © by Holt, Rinehart and Winston. Additions and changes to the original content are the responsibility of the instructor. Holt Physics 2 Section Quizzes Name:______________________________Class:__________________ Date:__________________ Heat continued _____ 5. What is the energy transferred to or from a unit mass of a substance during a phase change called? a. latent heat b. specific heat capacity c. internal energy d. thermal energy _____ 6. During a phase change, which of the following properties does not change? a. internal energy b. physical state c. temperature d. volume _____ 7. In a heating curve, what does a line with a positive slope indicate? a. the change in the substance’s state with added or removed energy b. the increase in the substance’s temperature with added energy c. the decrease in the substance’s temperature with added energy d. the change in the substance’s latent heat with added energy _____ 8. In a heating curve, what does a line with zero slope indicate? a. the change in the substance’s state with added or removed energy b. the increase in the substance’s temperature with added energy c. the decrease in the substance’s temperature with added energy d. the change in the substance’s specific heat capacity with added energy 9. Using the concept of specific heat capacity, explain why water remains cool on a hot day whereas the air above it becomes hot. _________________________________________________________________ _________________________________________________________________ _________________________________________________________________ _________________________________________________________________ _________________________________________________________________ 10. A metal part with a mass of 7.50 102 kg and a temperature of 93.0°C is placed in a calorimeter containing 0.150 kg of water. If the initial temperature of the water is 25.0°C, and the final temperature of the part and water 29.0°C, what is the specific heat capacity of the part? (cp,w = 4186 J/kg•°C) Original content Copyright © by Holt, Rinehart and Winston. Additions and changes to the original content are the responsibility of the instructor. Holt Physics 3 Section Quizzes





