Quinazoline & Quinoxaline Derivatives for Organic Electronics
advertisement

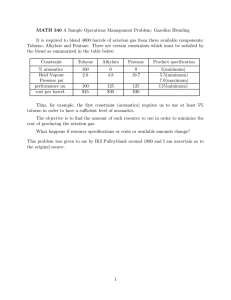
NEW QUINAZOLINE AND QUINOXALINE DERIVATIVES AS A BASE OF MATERIALS FOR ORGANIC ELECTRONIC DEVICES Moshkina T. N., Nosova E. V. Department of Organic and Biomolecular Chemistry, Chemical Technology Institute, Ural Federal University, 620002 Ekaterinburg, Mira Str. 19; +7(965)5263788; e-mail: tan.moshckina@yandex.ru -conjugated systems with push-pull architecture Project aim: design and synthesis of new organic chromophores as the basis of materials for electronic devices. Project tasks: - the synthesis and study of photophysical properties of V-shaped chromophores based on 2,3-bis(5-arylthiophen-2-yl)quinoxalines; - development of approaches to the functionalization of the new 2,4disubstituted quinazolines by introduction cyano-groups and donor substituents; estimation of NLO properties for new compounds; - the synthesis of novel BF2-complexes based on N,O-ligands; - carrying out the complex of physical and chemical investigations for the elucidation of the structure of molecules. Methods: Target chromophores have been synthesized through bromination at thenyl moiety or at position 4 of quinazoline core and subsequent palladium-catalyzed cross-coupling Suzuki reactions. BF2 complexes have been obtained by treatment of N,O-ligands with boron trifluoride etherate in a mixture of toluene and acetic acid. The structures of target compounds were confirmed by set of modern physical and chemical methods of analysis: 1Н, 13С, 19F, 11B NMRspectroscopy, mass-spectrometry as well as X-ray analysis. Photophysical properties were studied by absorption and fluorescence spectroscopy. The influence of solvent polarity on photophysical properties was investigated. The effect of protonation was studied by addition of TFA acid. The quantum yields of BF2 complexes in solid state were measured by the integrating sphere. The potential use of molecules as materials with NLO properties will be evaluated using the EFISH (electric field induced second harmonic generation) method in collaboration with French science group (Rennes Universiry). D D CN N S N N N S N N D S CN D R N N R 1 quinazoline 2 N R N R 3 quinoxaline Ph O X N NH R N N 5 R N F B O F F B O F R 4 R 5 N F B O F 4 Boron complexes based on N,O-ligands Results: BF2 complexes based on N,O-ligands V-shaped quinoxalines S NH2 + NH2 1 S 1. EtOH, t 2. NBS, DMF, r.t. 3. cross-coupling* O NH N NH N S 3a-d S N N * N * F cross-coupling* N N S N 3b Solvent 3a 3b 3c 3d toluene MeCN toluene MeCN toluene MeCN toluene MeCN Ar λem, nm 557 519 629 498 578 480 529 R 11a,b 11b R = H (a), t-Bu (b) N N * X O 3d 3c 6a *Cross-coupling: arylboronic acid or arylboronic acid pinacol ester, PdCl 2(PPh3)2, PPh3, K2CO3, toluene, EtOH, argon, 85 °C. λabs, nm 327, 377, 438 330, 369, 439 301, 424 364, 416 305, 354, 418 348, 409 330, 342, 389 341, 383 O N * Ar = * 3a S 6a,b 5 4 B F N Br R N CN CN S N Ar Ar =* 1. KCN, DMF, MePhSO3Na, 95 0C 2. NBS, DMF, 80 0C O O 2 O 2,4-disubstituted CN-containing quinazolines Ar Δvst, cm-1 3833 4317 8140 2739 7149 3721 7687 ϕ, % [a] 7 0 14 <1 8 2 11 5 6a toluene MeCN NH2 12 6a Solvent H λabs, nm 402 397 λem, nm 471 539 ϕ, %[a] <1 <1 N N R N R O Δvst, cm-1 3644 6636 AcONH4, I2 EtOH, 80 0C X + F B HO 13 R F R 14 R N HO O R 15a-d 12: X = H (a), Cl (b); 13: R = H (a), t-Bu (b); 14, 15: X = H, R = H (a); X = Cl, R = H (b); X = H, R = t-Bu (c); X=Cl; R = t-Bu (d). [a] 3-aminophthalimide in ethanol as standard. Br O O NH2 NH2 7 1. CuCl2, EtOH Br 2. POBr3, NEt3, toluene Ar cross-coupling* 8 HO N N + b-cyclodextrine N H2O, MeOH N N F HO 1 CN CN 9 N BF3(OEt)2, AcOH, toluene, t NH2 N O N 16 N H + [a] 3-aminophthalimide in ethanol as standard. Absorption (a) and emission (b) color changes 6b BF3(OEt)2, AcOH, toluene, t X 17 NH2 18 B O F 10a-c CN Ar = N * N * N * S 10a N *Cross-coupling: arylboronic acid or arylboronic acid ° PdCl2(PPh3)2, PPh3, K2CO3, toluene, EtOH, argon, 85 C. N 3a 3b a b without TFA (1) 135 equiv of TFA (2) 3500 equiv of TFA (3) 6a S a b without TFA (1) 7000 equiv of TFA (2) 10b 10c pinacol 15c ester, N Possible practical applications Changes in the emission spectra of toluene solution of 3a upon the addition of TFA The studied compounds are of great value for further application as materials for optical devices. Such materials have potential applications in modulation of optical signals, medicine, spectroscopic and electrochemical sensing, microfabrication and imaging, laser technology, data storage, telecommunication etc. 11a 11b 15a 15b 15c 15d 18 15d λabs, nm 354 366 386 395 410 420 407 18 toluene λem, ϕ, nm %[a] 423 24 441 42 529 1.3 542 0.8 565 0.2 552 0.1 530 8.6 Δvst, cm-1 4608 4647 6607 6866 6691 5694 5702 solid state λabs, ϕ, nm % 500 18.5 469 66.3 502 0.2 500 11.1 541 1.9 540 2.5 - [a] quinine sulfate in 0.1 M H2SO4 as standard 11a 11b 15a 15b 15c 15d 18