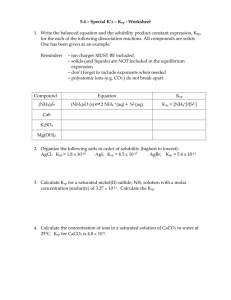
Infinite Chemistry Ion – Precipitate Equilibrium ith Solubility Product(Ksp) Ionic compound consist of ..................................... and .................................... that are electrostatically attracted to each other strongly leading to formation of a ........................................ lattice structure. Dissolution of an ionic substance in a polar/nonpolar solvent like H2O causes the ions to separate from each other and shift into the solution as mobile/stationary, solid/aqueous ions. Eg : NaCl(s) ------> ............... + ............... MgCl2(s) ------> ............... + ............... Certain ionic compound/salts are highly soluble in water. Eg : He ra th CaCO3(s) ------> ............... + ............... Other salts are almost insoluble, which means only a small/large portion is dissolved and turn into ch aqueous ions. These are known as sparingly soluble salts and produce a reversible reaction that gain eventually gain .................................... AxBy(aq) x A (aq) + y B (aq) Y+ X– Sachith Herath B.Sc.(Hon’s) Page 1 Sa Exercise : Write Ksp expressions for the following ionic compounds AgCl, Ag2CrO4, Ca(OH)2, BaSO4 etc. Infinite Chemistry ith Saturated Solutions A solution with a sparingly soluble ionic compound in equilibrium with it’s aqueous ions contains constant concentrations of those 2 types of ions. Therefore, as long as the temperature is fixed, no more compound can be further dissolved and hence the solution is said to be saturated. Asaturated solution can be made in two ways. 1. By dissolving the solid ionic compound. Here the concentrations of the two ions will be in ...................................... ratio. 2. By mixing 2 solutions containing the cation and the anion. Here, the concentration of the 2 ions can differ from the stoichiometric ratio. eg: Cation concentration can be much greater than that of the anion. y+ x x– y y+ ch If [A (aq)] [B (aq)] < Ksp then... – the solution is saturated/unsaturated. He ra th In either of the cases, the saturatedness can be determined with reference to the following conditions. x x– y x x– y If [A (aq)] [B (aq)] = Ksp then... – the solution is saturated/unsaturated but a precipitate will not visible/invisible. y+ Problems Based on Ksp Calculation of Ksp when the solubility is given. 2. Calculation of Solubility when Ksp is given. 3. Common ion effect. Sa 1. Sachith Herath B.Sc.(Hon’s) Page 2 If [A (aq)] [B (aq)] > Ksp then... – the solution is saturated/unsaturated and a precipitate will be visible/invisible.