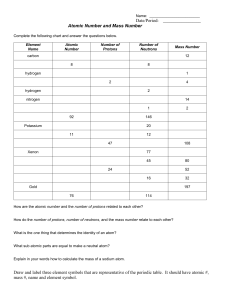
CHEMISTRY 1 Atomic Properties Activity Name: _____________________________ In today’s class you will be exploring some of the properties of various elements. These properties will include: atomic symbols, atomic numbers, numbers of protons, numbers of electrons, number of neutrons, mass numbers, net charges, and the stability of the isotopes. To do so, you will be using an online applet created and available for free through the University of Colorado. 1. Go to the following website: https://phet.colorado.edu/sims/html/build-an-atom/latest/buildan-atom_en.html . You’ll see a screen that looks like this 2. Select the “Atom” option to open up the first section of the applet that you will use. You will find a screen like this 3. On this page you will want to turn on a few options before you begin. Open the “Net Charge” and “Mass Number” boxes on the right hand side, and select the “Stable/Unstable” option at the bottom. Drag one proton to the center of the atom. After doing so your screen should look like this: 4. From this screen you will be able to determine all of the information in regard to several different atomic arrangements. You’ll find those arrangements beginning on the next page. Add protons, neutrons, and electrons to the atoms by clicking and dragging the spheres from their respective containers into the atoms. Remove them by dragging them from the atom into their containers. 5. You can also switch between the “Orbits” view and “Cloud” view by toggling just above the electrons bucket. You’ll need to do this several times throughout the activity. 6. When you’ve finished the activity beginning on the following page, click the home icon at the bottom, then on the right side of the screen double click the “Game” image. There you will be taken to a screen like this: 7. Choose the first option and follow the directions for the game. Once finished, raise your hand and I will come over and record your score for that game. Then return to the screen above and continue through all of the games. Each time you finish a game raise your hand and I will come by to record your score. When you’ve finished with all 4 games you have finished the in-class activity. The questions to answer in this packet are due Monday. Questions to Answer Make predictions based on the following information. Then use the atom model to check if your predictions were correct. Atomic structure: 3 protons, 3 neutrons, 4 electrons Prediction Actual Atomic Number Atomic Symbol Net Charge Mass Number Stable/Unstable Atomic structure: 2 protons, 2 neutrons, 1 electron Prediction Actual Atomic Number Atomic Symbol Net Charge Mass Number Stable/Unstable Atomic structure: 8 protons, 8 neutrons, 10 electrons Prediction Atomic Number Atomic Symbol Net Charge Mass Number Stable/Unstable Actual What is a rule for making a neutral atom? ___________________________________________________________________________________ What is a rule for making a +ion? ___________________________________________________________________________________ What is a rule for making a – ion? ___________________________________________________________________________________ What is a rule for determining the mass of an atom? ___________________________________________________________________________________ Using all of your rules, figure out what changes for each of these actions to an atom or ion. You can test your ideas with the simulation. If you have new ideas, rewrite your rules. Action Add a Proton Action Remove a Neutron Action Remove an Electron Action Add an Electron What Changes? □ Element □ Charge □ Mass What Changes? □ Element □ Charge □ Mass What Changes? □ Element □ Charge □ Mass What Changes? □ Element □ Charge □ Mass How Does it Change? How Does it Change? How Does it Change? How Does it Change?