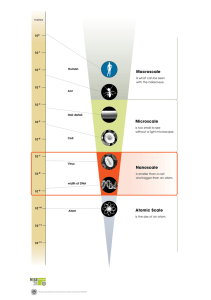
The Structure of an Atom Label the diagram of an atom with the following words: Electron, Orbit, Proton, Neutron, and Nucleus. - + + + - Fill in the missing words in the sentences below: The subatomic particles which make up atoms are called and called the , . and are contained in the centre of the atom, which is . orbit around the are positively charged, . are negatively charged and are neutral. Answer the following questions: 1. How is the atomic number of an element determined? 2. If an atom is neutral, what does that say about the subatomic particles in the atom? What does the picture below show? - - - + - + + + + + + - How many protons does the atom have? _______________ How many neutrons does the atom have? _______________ How many electrons does the atom have? _______________ What is the atomic number of this atom? _______________ What is the name of this atom? _______________ What is the symbol for this atom? _______________ Is this a neutral atom? Explain your choice. In the space below, draw an atom with 5 protons, 6 neutrons and 2 electrons. What is the atomic number of this atom? _______________ What is the name of this atom? _______________ What is the symbol for this atom? _______________ Is this a neutral atom? Explain your choice.