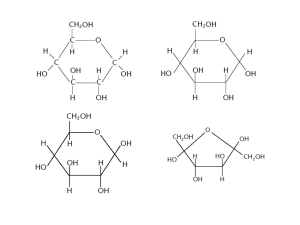
3 - Distinguish between monosaccharides, disaccharides and polysaccharides (glycogen and starch – amylose and amylopectin) and relate their structures to their roles in providing and storing energy (β-glucose and cellulose are not required in this topic). Carbohydrates! Carbohydrates are a major class of biological molecule composed of C, H, and O atoms, usually in the ratio 1:2:1. They are a major source of energy for metabolism and an important structural component. The simplest carbs are sugars (saccharides). Simple sugars are linear, or far more commonly, cyclic molecules containing 5 or 6 carbon atoms. Glucose Galactose Fructose Monosaccharides with 6 carbon atoms! Product of photosynthesis Same chemical formula, C6H12O6 – differ only in arrangements of their Found in dairy products atoms/ configuration. Look at the Commercial sweetener diagram below! A monosaccharide is a simple sugar; an unbranched carbon chain with either an aldehyde (HC=O) or a ketone (C=O) group. Almost all monosaccharides in cells are in ring form. For example, cyclic glucose is formed when the oxygen atom of the hydroxyl group on carbon 5 forms a covalent bond with carbon 1, which is part of an aldehyde group. Combining two monosaccharides together forms a ….disaccharide! For example, sucrose (table sugar) is a disaccharide that combines one molecule of glucose and fructose. Joining many monosaccharides together forms a complex polymer called a polysaccharide. Examples include starch and glycogen, which are both very important for long-term energy storage, or cellulose in plant cell walls, which is very important for structural support. 3 - Distinguish between monosaccharides, disaccharides and polysaccharides (glycogen and starch – amylose and amylopectin) and relate their structures to their roles in providing and storing energy (β-glucose and cellulose are not required in this topic). GLYCOSIDIC BONDS!! Monosaccharides are attached to each other by covalent bonds called glycosidic bonds (Fig. 2.24). As with peptide bonds, the formation of glycosidic bonds involves the loss of a water molecule. A glycosidic bond is formed between carbon 1 of one monosaccharide and a hydroxyl group carried by a carbon atom in a different monosaccharide molecule. Polysaccharides are ideal for storing carbohydrates because… 1. They can form very compact molecules, ideal for storing carbohydrates in cells. For example, the angle of amylose’s 1,4 glycosidic bonds results in it having a compact, coiled structure which is ideal for storage because you can fit more in to a small space. 2. They are insoluble so have no effect the osmotic pressure inside the cell. 3. Non-reducing (chemically and physically inactive) so storing them doesn’t interfere with the other functions of the cell. 4. They are easily broken down to small glucose monomers (monosaccharide unit) by a hydrolysis reaction which provides an instant supply of energy for cells. 3 - Distinguish between monosaccharides, disaccharides and polysaccharides (glycogen and starch – amylose and amylopectin) and relate their structures to their roles in providing and storing energy (β-glucose and cellulose are not required in this topic). Polysaccharides 1. Starch – the main energy storage in plants Starch is a polysaccharide made of many alpha glucose molecules arranged into two different structural units: amylose and amylopectin. Looking at the relationship between structure and function of: Amylose: Amylose contains glucose molecules joined mainly by alpha 1-4 glycosidic bonds. Alpha 1-4 glycosidic bonds result in unbranched chains, which form a coiled, helical structure. This makes it compact and thus ideal for storage in cells because you can fit more glucose molecules into a smaller space. Amylopectin: Amylopectin also contains glucose molecules joined by alpha 1-4 glycosidic bonds, but it also contains many more alpha 1-6 glycosidic bonds. The alpha 1-6 glycosidic bonds result in amylopectin having a highly branched structure; these side branches have lots of glucose molecules that can be broken off rapidly when energy is needed. Summary: More about structure and function: Starch is also insoluble in water, so it doesn’t affect water relations and osmosis in a cell. 3 - Distinguish between monosaccharides, disaccharides and polysaccharides (glycogen and starch – amylose and amylopectin) and relate their structures to their roles in providing and storing energy (β-glucose and cellulose are not required in this topic). 2. Glycogen Animal cells do not store excess glucose as starch but as Glycogen. Glycogen has a similar structure to amylopectin (has both alpha 1–4 glycosidic bonds and 1–6 glycosidic bonds) but it contains more alpha 1–6 glycosidic bonds that produce an even more branched structure. More branches mean that the stored glucose can be broken down more rapidly, which is important for energy release in animals and indicates the higher metabolic requirements of animals compared with plants. It’s also a compact molecule leading to more glucose molecules in a smaller space in a cell. Like starch, glycogen is also insoluble in water, so it doesn’t affect the osmosis and water relations in a cell. It is also a large molecule, so it stores a lot of energy. Describe the structure of starch and explain why this structure makes it a suitable molecule for storing energy. [4 marks]. Starch is a polysaccharide made of many alpha glucose molecules arranged into amylose and amylopectin (1). Amylose consist of glucose molecules joined together by 1,4 glycosidic bonds (1) which result in a long, unbranched chains which form a spiralled, coiled structure that makes amylose a very compact molecule (1) which is ideal for storage because more glucose can be stored in a smaller space in the cell (1). Amylopectin also has 1, 4 glycosidic bonds but also 1, 6 glycosidic bonds (1) which result in highly branched chains; these side branches have a lot of glucose molecules which can be rapidly broken off when energy is needed. Starch is also insoluble (1) so it has very little osmotic effect (1). Describe the structure of glycogen and explain why it is a suitable molecule for storing energy. [4 marks] Glycogen consist of alpha glucose molecules (1) joined by 1, 4 and many more 1, 6 glycosidic bonds (1). These 1, 6 glycosidic bonds result in glucose having a highly branched structure (1); these side branches mean there’s more stored glucose that can be rapidly broken off when energy is needed. Glycogen also has a compact structure (1) leading to more glucose in a smaller space in the cell (1). Glycogen is also a large molecule and so stores a lot of energy. Moreover, it has LOW solubility (1) and so does not affect the osmotic pressure of the cell, making it ideal for storage. Note: don’t go like ‘glycogen has 1, 4 and 1, 6 glycosidic bonds. Mention that it’s a GLUCOSE MOLECULE joined by these bonds.