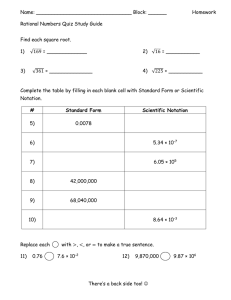
Name____________________________________________________________Period_____Date__________________ PAP Chemistry Homework HW # 1 Copying is not allowed! 1. Write each of the following numbers in exponential notation: a) 531,100 __________ b) 0.00000003753 __________ c) 64300 __________ d) 0.0033165 __________ e) 0.08316 __________ f) 9335 __________ 2. Convert each of the following numbers in exponential notation to conventional decimal form: a) 2.234 x 10-5 ______________ d) 2.935 x 10-3 ______________ b) 9.33 x 102 ______________ e) 7.35 x 10-2 ______________ c) 4.230 x 104 ______________ f) 8.138 x 10-12 ______________ 3. Rewrite each number with a coefficient between one and ten (in other words, write each number in CORRECT scientific notation: a) 3275 x 103 ______________ d) 433.9 x 10-1 ______________ b) 92 x 10-4 ______________ e) 0.01365 x 10-2 ______________ 5 c) 0.6311 x 10 ______________ f) 0.0641 x 106 ______________ 4. How many significant figures are in each of the quantities listed below: 5. a) 4354 mg __________ d) 0.06380 km __________ g) 0.153336 g __________ b) 0.03353 L __________ e) 3310.0 mL __________ h) 0.00360 g __________ c) 532.20 mL __________ f) 3 x 107 kg __________ i) 1.8398 x 10-3 g __________ j) 25300 m __________ Round off the given quantity 37.7358064 to the number of significant figures indicated: one ____________________ three _____________________ five _____________________ two _____________________ four _____________________ six 6. _____________________ Perform the indicated calculation and express the answer in the correct number of significant figures: 0.370 843 0.0704 _____________________ 0.0042 17.10 7. Complete the following operations and round off the answers to the proper number of significant figures: a) 8. 18 .7 - 0.56 _____________________ b) 1.59 10 -4 - 6.42 x 10 -5 _____________________ Round off the quantity 34.1036738 to the number of significant figures indicated: one _____________________ three _____________________ five _____________________ two 9. _____________________ four _____________________ six _____________________ Express each of the following in correct scientific notation with the correct number of significant figures: a) (5.68 x 10-4) x (6.532 x 104) _______________ b) (7.5333 x 105) x (1.56 x 10-9) _______________ 10. Express each of the following in correct scientific notation with the correct number of significant figures: a) (6.7334 x 10-8) / (2.34 x 106) _______________ b) (1.345 x 109) / (9.35 x 10-7) _______________