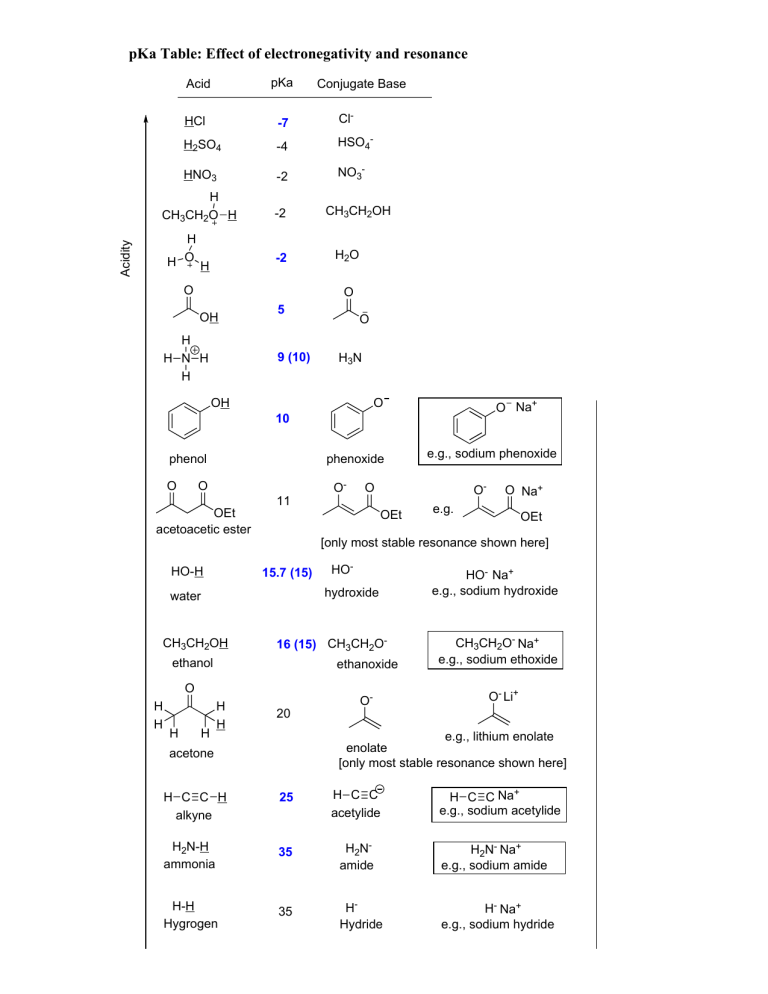
pKa Table: Effect of electronegativity and resonance Acid pKa HCl -7 Cl- H2SO4 -4 HSO4- HNO3 -2 NO3- H CH3CH2O H -2 Acidity H H O H -2 O Conjugate Base CH3CH2OH H2O O OH H H N H H 5 9 (10) O H3N OH O O Na+ 10 phenol O phenoxide O OEt acetoacetic ester HO-H 11 15.7 (15) OEt HO- 16 (15) CH3CH2O- ethanol ethanoxide O H O- hydroxide CH3CH2OH H O H H O Na+ e.g. OEt [only most stable resonance shown here] water H H O- e.g., sodium phenoxide 20 O- HO- Na+ e.g., sodium hydroxide CH3CH2O- Na+ e.g., sodium ethoxide O- Li+ e.g., lithium enolate enolate [only most stable resonance shown here] acetone H C C Na+ e.g., sodium acetylide H C C H alkyne 25 H C C acetylide H2N-H ammonia 35 H2Namide H2N- Na+ e.g., sodium amide H-H Hygrogen 35 HHydride H- Na+ e.g., sodium hydride 44 alkene CH3CH2CH2CH2-H Butane 60 CH3CH2CH2CH2Butyl carbanion CH3CH2CH2CH2- Li+ e.g., butyl lithium Basicity H H C C H H