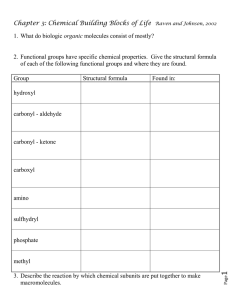
BIOLOGICAL MOLECULES INTRODUCTION Everything in the physical universe is matter There are two main types of matter: Living things/matter and Non-living matter BUILDING BLOCKS OF MATTER Matter is made up of three main types of building blocks: Atoms Ions molecules BIOLOGICAL MOLECULES They are molecules that make up living things They form the building blocks of living things WHAT ARE BIOLOGICAL MOLECULES MADE OF? They are made of atoms of the same element or different elements ORGANISATION OF ORGANISMS ELEMENTS THAT MAKE UP BIOLOGICAL MOLECULES The main elements that make up the molecules of living things include: Carbon Hydrogen Oxygen nitrogen The four main elements combine to form organic substances that make up living organisms ORGANIC SUBSTANCE It is one whose molecules contain carbon and hydrogen TYPES OF ORGANIC SUBSTANCES Organic substances may be: Monomers or polymers MONOMERS They are small molecules that are joined to make large molecules (polymers) by chemical reactions POLYMERS They are very large molecules formed by joining two or more small molecules (monomers) NOTE Thus, large molecules are made from smaller molecules TYPES OF BIOLOGICAL MOLECULES FOUND IN LIVING ORGANISMS Biological molecules found in living organisms include: Carbohydrates Proteins Fats and oils/lipids Nucleic acids Vitamins water CARBOHYDRATES They contain carbon, hydrogen and oxygen only They have the general formula Cx(H2O)y where x and y are whole numbers TYPES OF CARBOHYDRATES There are three main types of carbohydrates, namely: Monosaccharides Disaccharides polysaccharides NOTE “Saccharide” means “sugar” or “sweet substance” MONOSACCHARIDES They are also known as simple sugars They are the simplest carbohydrates They are the monomers that can be joined to form all the other types of carbohydrates They are sweet tasting They readily dissolve in water EXAMPLES OF MONOSACCHARIDES Glucose Fructose Galactose Ribose and deoxyribose DISACCHARIDES They are formed when two monosac- charides are joined together They contain two carbon rings They are also soluble in water They have a sweet taste POLYSACCHARIDES They are formed when many glucose or monosaccharide molecules are joined together They are large molecules containing a large number of monosaccharide molecules in a long chain They are not soluble in water They do not have a sweet taste TEST FOR CARBOHYDRATES BENEDICT’S SOLUTION TEST FOR REDUCING SUGARS NOTE Reducing sugars include glucose, fructose, galactose, maltose and galactose IODINE SOLUTION TEST FOR STARCH USES OF CARBOHYDRATES IN LIVING ORGANISMS Glucose is broken down in respiration by cells to release energy They are used for the storage of energy for cells Cellulose is used to make plant cell walls very strong which helps to maintain the shape of the cell NOTE Plants store carbohydrates as STARCH Animals store carbohydrates as GLYCOGEN These can easily be changed back to glucose and used for respiration FATS AND OILS Fats and oils are also known as lipids They also contain the elements, carbon, hydrogen and oxygen only They are made of the monomers: Glycerol Fatty acids One glycerol and three fatty acids are joined to form a lipid molecule Fats are not soluble in water Fats are solid and oils are liquid TEST FOR FATS AND OILS (LIPIDS) THE ETHANOL EMULSION TEST FOR FATS USES OF FATS AND OILS/LIPIDS IN LIVING ORGANISMS They can be respired by cells to release energy, producing twice as much energy as carbohydrates They are used for the storage of energy for cells The fat store below the skin of humans act as an insulator against heat loss Lipids form part of the cell membrane and the internal membranes of the cell such as the nuclear membrane NOTE Fat is stored in specialized cells under the skin called adipose cells A layer of adipose cells under the skin forms the adipose tissue PROTEINS They contain the elements carbon, hydrogen, oxygen, nitrogen and sometimes sulphur They are made up of monomers called amino acids Some proteins are soluble in water, others are not soluble in water HOW PROTEINS ARE FORMED Proteins are formed when many amino acid units are joined end to end There are about 20 different amino acids The long chain of amino acids which form proteins can coil up into different shapes The way the amino acid chain coils up also determines the 3D shape of the protein molecule The specific order of the amino acids joined determines the shape of the protein Different sequences of amino acids give different shapes to protein molecules PROTEIN SHAPE AND FUNCTION The shape of a protein determines its function For example the shape of an enzyme creates an active site with a specific shape which determine the reactions of the enzyme The shape of an antibody creates a binding site with a particular shape that can destroy a particular bacterium or virus TEST FOR PROTEINS THE BIURET TEST FOR PROTEINS OR USES OF PROTEINS IN LIVING ORGANISMS They are used to make enzymes They are used to make important parts of cell membranes, mitochondria and ribosomes They are used to make antibodies which destroy bacteria and viruses that enter the body They are used to make haemoglobin in red blood cells WATER Most cells contain about 75 – 80% water Cells die if the water content falls below this The cytoplasm is a solution of water and dissolved substances The spaces between cells is also filled with a watery fluid In the cells of all organisms, chemical reactions go on all the time to sustain life The reactions that take place in cells all the time are called metabolism Metabolic reactions require water in order to occur IMPORTANCE OF WATER It serves as a solvent in which metabolic reactions take place It forms an important part of blood plasma It is needed to dissolve enzymes and nutrients in the digestive system so that digestion can occur It is needed to get rid of waste products such as urea by the kidneys VITAMINS They are organic molecules that are needed by cells in small amounts to regulate metabolic reactions TEST FOR VITAMINS USE OF VITAMINS IN LIVING ORGANISMS They play a very important metabolic reactions in cells and their absence hinders the cell’s reactions STRUCTURE OF DNA DNA stands for DeoxyriboNucleic Acid DNA is the chemical that makes up genes and chromosomes It is the material inherited from parents which gives us our characteristics It is made up of monomers called nucleotides A DNA molecule is made up of two long strands of nucleotide, coiled/twisted together to form a spiral called a double helix Each strand is made up of monomers called nucleotides The bases on the two opposite strands are held together by bonds which form crosslinks There are four kinds of bases which are adenine (A), thymine (T), cytosine (C) or guanine (G) The bases always pair up in the same way: A with T and C with G HOW DNA IS USED AS A TOOL FOR CLASSIFICATION When molecules of DNA are used to classify species, only one of the two DNA strands is sequenced First the DNA sequence from one strand of a DNA molecule from each species is lined up against one strand from another species The bases of the DNA sequences from the same strand can then be compared with each other The number of differences between the DNA sequences of the species are counted and recorded The most closely related species have the fewest differences between their DNA sequences DNA BARCODING It is the sequencing of the DNA of one particular gene to help identify different species of organisms The sequences found are called barcodes IMPORTANCE OR FUNCTIONS OF DNA The sequence of the bases in DNA provides a code that determines the sequence of amino acids in a protein molecule and hence the characteristics of an organism It codes for specific proteins It stores genetic information It can be copied to pass on information to new cells It forms genes which determine the characteristics of organisms END OF CHAPTER