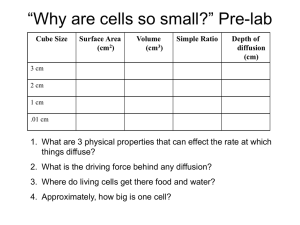
17.09.2014 1 Irena Sionek 2IB DIFUSSION IN AGAR CUBES – LAB REPORT Research question What is the effect of surface area on volume ratio on the rate of diffusion of pigment from the agar cube measured by percentage volume of diffusion over time? Background Diffusion is a process of spontaneous spreading of particles in an environment (e.g. a cell), which is a consequence of chaotic collisions of molecules of the diffusing substances with each other or with the molecules of the surrounding environment. It can be observed in cells when substances e.g. water, oxygen, nutrients, cellular waste are transported inside, outside the cell or to different cellular organelles. It is easy to observe diffusion by gently pouring a coloured liquid into water. In this experiment I will examine diffusion of a solution in a jelly-like substance - agar. Because both the solution and the agar are colourless I will use agar that had before been mixed with phenolphthalein. This also colourless substance is a commonly used indicator that changes its colour into pink with the presence of a base. It will turn pink immediately when mixed with a NaOH solution. When the agarphenolphthalein cubes will be put into the sodium hydroxide solution, NaOH will start diffusing through the agar cubes and turn the inside of the cube pink gradually. We want to examine whether ad how the rate of diffusion over time is dependent on the cubes surface area to volume ratio (SA:V). Smaller cubes have bigger SA:V than big cubes. We know that e.g. cells seek to have the biggest surface area possible and a small volume. We will therefore see, whether one of the reasons they do it is the rate of diffusion. Hypothesis If a big surface area to volume ratio is something very desired by cells that need efficient and fast diffusion for their functioning (transporting nutrients, oxygen, water, waste), then the greater the SA:V, the greater is the rate of diffusion of pigment from the agar tube measured by the percentage volume of diffusion over time. 17.09.2014 2 Irena Sionek 2IB Variables: dependent: independent: controlled: the distance from the core of the pigment to the border of the agar cube the size of the agar cube (edge length) , this will be: 1 cm, 1.5 cm, 2 cm, 2.5 cm time type of solution in which we submerge – 4% sodium hydroxide Temperature of the solution: 21 ° C Time we keep the cubes in the solution: 10 minutes Volume of the solution – 100 cm3 (± 0.5 cm3) The material used to prepare agar cubes The method of measuring the length of cube edges Controlling the variables Type of solution The solution temperature Time we keep the cubes in the solution Volume of the solution The material used to prepare the cubes The method of measuring the length of cube edges We put exactly 4g of sodium hydroxide in 96 cm3 of water. We measure the NaOH with a weight and the volume of water with a measuring cylinder. We measure it with a thermometer with a proper error and control it with a water bath set at that exact temperature (21 ° C). We put the beakers in the bath. We measure this with a timer (± 0.5 s) We use a measuring cylinder (one of a corresponding but bigger volume), (± 0.5 cm3) We use the same package of agar and same of the phenolphthalein. It was bought at a chemical store and the package was labelled. We use the same ruler for all 17.09.2014 3 Irena Sionek 2IB Apparatus and materials (one of each for a group) Knife Ruler Beaker Measuring cylinder Timer Spoon Petri dish Weight Paper towels (optional) 96 cm3 water 4 g NaOH Tray of agar with phenolphthalein Method We have 6 groups making the very same experiment, so we have 6 trials. This gives the experiment reliability. We already have a tray of agar with phenolphthalein. The instructions are for one group. 1) Measuring out with a ruler cut out 4 agar cubes with the following edge lengths: 1cm; 1.5 cm; 2 cm; 2.5 cm. Cut as accurately as possible. 2) Pour 96 cm3 of water into the beaker. 3) Weight out 4g of sodium hydroxide and add it to the water in the beaker. 4) Put the four cubes into the solution. 5) Immediately turn on the timer. 6) Leave the cubes in the solution for 10 minutes; remember to check on the temperature. 7) After the 10 minutes had passed, take out the cubes onto the petri dish using a spoon. 8) If necessary blot the cubes with a paper towel, so no sodium hydroxide is left on them. 9) Cut each of the cubes in half and collect measurements using a ruler. Safety As NaOH is a corrosive acid that can cause chemical burns on skin and permanent blindness when contacted with eyes. Be sure to wear gloves all of the time and do not touch your eyes. 17.09.2014 4 Irena Sionek 2IB Collecting raw data I will use this table to record the collected data: DIMENSION (cm ± 1 mm) SURFACE AREA (Cm2) INITIAL VOLUME without the violet colour (Cm3) FINAL VOLUME Without the violet colour (Cm3 ± 0.5 mm3) DIFFUSED VOLUME - With violet colour (Cm3) Group number 1 2 3 4 5 6 1 2 3 4 5 6 1 2 3 4 5 6 1 2 3 4 5 6 17.09.2014 5 Irena Sionek 2IB Data processing To do all the mathematical operations and draw graphs I used a simple calculator and Microsoft Excel 2011. These are the used formulas and example operations. Calculating the surface area of cubes e – edge of the cube SA – surface area 𝑺𝑨 = 𝟔 𝒆𝟐 E.g. �� (1)1 = 6 × 12 = 6 Calculating the initial volume V- volume 𝑽 = 𝒆𝟑 e.g. 𝑉(1) = 13 = 1 Calculating the final volume fV – final volume w – edge of the little uncoloured cube in the centre of the whole agar cube 𝒇𝑽 = 𝒘𝟑 e.g. 𝑓𝑉 (1) = 03 = 0 Calculating the diffused volume dV – diffused volume dV = V – fV e.g. dV (1) = 1 – 0 = 1 by (1) i will mark the smallest cube (1cm x1cm x1cm) and the biggest one will respectively be marked (4) 1 17.09.2014 6 Irena Sionek 2IB DIMENSION (cm ± 1 mm) 1x1x1 1.5x1.5 x1.5 2x2x2 2.5x2.5x2.5 SURFACE AREA (cm2) 6 13.5 24 37.5 INITIAL VOLUME without the violet colour (cm3) 1 3.375 8 15.625 FINAL VOLUME Without the violet colour (cm3 ± 0.5 mm3) DIFFUSED VOLUME - with violet colour (cm3) 0 1 12 0 1 2 0 1 3 0 1 4 0 1 5 0.216 0.784 6 0 3.375 1 0 3.375 2 0 3.375 3 0 3.375 4 0 3.375 5 0.729 2.646 6 0.064 7.936 1 0.125 7.875 2 0.008 7.992 3 0.512 7.488 4 0 8 5 4.096 3.904 6 1.331 14.294 1 0.343 15.282 2 0.216 15.409 3 1.728 13.897 4 1.331 14.294 5 12.167 3.458 6 Anomalies 2 group 1 is the group in which I personally worked. group number 17.09.2014 7 Irena Sionek 2IB The highlighted and bolded data (collected by group 6) seems to be anomalous. It visibly differs from the data collected by other groups (from which most are very similar). For example let’s look at the data collected from the biggest cube – the diffused volume. While data collected by the groups 1-5 stays in the interval 13.897 – 15.409 [cm3], the number collected by group 6 is 3.458 cm3, which is significantly deviating. It is most probable that the reason of such an inconsistency is an incorrect conduct of the method. We can presume that the time the cubes stayed in the solution wasn’t exactly 10 minutes (maybe because of a broken timer) or the ruler used to take measurements was much different from the ones used by other groups. The time between taking out the cubes from the solution and measuring can impact the results. Therefore, for the good of the experiment’s result I decided to skip the data collected by group 6 in the data processing. Calculating the surface area to volume ratio and diffusion rate in each of the groups (excluding group no. 6) SA:V – surface area to volume ratio 𝑺𝑨: 𝑽 = 𝑺𝑨 𝑽 e.g. 𝑆𝐴: 𝑉 (1) = 6 1 =6 %Vd – percent volume of diffusion %𝑽𝒅 = 𝒅𝑽 × 𝟏𝟎𝟎 𝑽 e.g. %𝑉𝑑 (1) = 1 1 × 100 = 100 17.09.2014 8 Irena Sionek 2IB DIMENSION (cm ± 1 mm) 1x1x1 1.5x1.5 x1.5 2x2x2 2.5x2.5x2.5 SURFACE AREA TO VOLUME RATIO 6 4 3 2.4 PERCENT VOLUME OF DIFUSION over 10 minutes [%] group number 100 1 100 2 100 3 100 4 100 5 100 1 100 2 100 3 100 4 100 5 99.2 1 98.4 2 99.9 3 93.6 4 100 5 91.5 1 97.8 2 98.62 3 88.94 4 91.48 5 17.09.2014 9 Irena Sionek 2IB correlation between SA:V and % volume of diffusion % volume of diffusion [%] 102 100 98 group 1 96 group 2 94 group 3 group 4 92 group 5 90 88 0 1 2 3 4 5 6 7 SA:V Calculating the average from % volume of diffusion in each of the cube sizes. A – average %Vd in this cube size Σ𝑥 - sum of all the %Vd in all of the groups n – number of the groups 𝑨= A (1) = 𝚺𝒙 𝒏 100 + 100 + 100 + 100 + 100 = 100 5 A (2) = 100 A (3) = 98.22 A (4) = 93.668 17.09.2014 10 Irena Sionek 2IB Calculating the standard deviation of mean % volume of diffusion in each of the cube sizes σ – standard deviation x – each value of A in each of the cube sizes n – number of values of A in this cube size 𝑥̅ – mean of all values of A in this cube size 𝝈= 𝚺 (𝒙 − 𝒙̅ )𝟐 𝒏−𝟏 σ (1) = 0 σ (2) = 0 σ (3) = 2.661203 σ (4) = 4.284777707 Correlation between SA:V and average % volume of diffusion in each of the cube sizes Correlation between SA:V and mean % volume of diffusion mean % volume of diffusion 102 100 98 96 94 92 90 88 0 1 2 3 4 SA:V 5 6 7 17.09.2014 11 Irena Sionek 2IB Conclusion From the data obtained it can be concluded that there is some relationship between SA:V and the rate of diffusion. The experiment results seen on the graph (trend line) show that the bigger is the surface area to volume ratio, the bigger is the percentage volume of diffusion, so the rate of diffusion of pigment from the agar cube. Looking at the data we can notice that smaller cubes (of smaller volume) have bigger SA:V. We can than state that the results of the experiment have also shown, that the smaller is the cube, the bigger is the rate of diffusion. We can see that differences in the diffusion rate are much bigger in bigger 3 cubes (4 % difference in the % volume of diffusion with the difference of only 0.6 in their SA:V). There was no noticeable difference in the diffusion rate of the smaller4 cubes ( 0% difference in the diffusion rate with the difference of 2 in their SA:V). The graph appears to flatten off at the point of the cube with the dimensions 1,5cm x 1,5 cm x 1,5 cm and SA:V = 4 and stays at the same level to the smallest cube (1 cm x 1 cm x 1cm, SA:V = 6). The visible trend is called decelerating increase. The relationship between the variables has its explanation. Bigger surface area to volume ratio means that there is more surface through which the substance (NaOH in this case) has to pass , so more compounds can do it at once and less volume that they have to diffuse through in order to reach the cube’s centre. There are more of the compounds and also each of them has much less to do, so they do their “work” faster. The obtained results have a reflection in the biological world. As said in the “background” section” cells seek to have the biggest SA:V they can. Cells need to take in nutrients from their surroundings and expel waste through their cell membrane. If there is a large surface area to volume ratio, there is more cellular membrane to take care of both of these functions. If a cell gets too large, it’s SA:V gets smaller and there is not enough surface area to expel wastes or intake nutrients (the cell has bigger volume so needs more of them) fast enough and the cell shrinks or dies. by bigger I mean the ones with SA:V = 2,4 and 3 (2.5cm x2.5cm x2.5cm and 2cm x2cm x2cm) 4 by smaller I mean the ones with SA:V = 4 and 6 (1.5cm x1.5cm x1.5cm and 1cm x1cm x1cm) 3 17.09.2014 12 Irena Sionek 2IB Evaluation Overall I am not very confident about the conclusion I made, as there were certain points in this experiment that may cause objections. I don’t find any mistakes in the chosen apparatus and materials. The first thing, which contributed to the low reliability of these experiment is a small, insufficient number of trials. With only 6 groups conducting the experiment, when one of them makes an error it has a significant impact on the final results of the whole experiment as the group’s result states 1/6 (about 17 %) of the final result. Second of all, it was already mentioned that the data collected by group number 6 is very different from the data collected by the other groups. It is very probable that this group has misunderstood the method. Having calculated the standard deviation of mean % volume of diffusion we can see (it’s shown on the graph – standard deviation error bars) that the value of standard deviation is very big in two of the biggest cubes, even more than 4 in the case of the biggest cube. Both the odd results of group 6 and the high value of standard deviation can be the result of other human errors. Keeping the cubes in the solution much more or much less than 10 minutes has probably affected the results most significantly – the diffusion would be at a different stage. The same happens with waiting too much between removing the cubes from the solution and taking measurements. Thirdly, what can have some effect on the low reliability is that different people were taking measurements using rulers with different errors. Uncertainties themselves might have affected the results only on a very small scale as they all had a small range. Considering the weaknesses of this experiment we should avoid repeating the same mistakes next time. First of all, it is better when much more trials are conducted (preferably tens), but the same person, using only one ruler with a specific error, does them all. In this case the error would have the same impact on all of the data, so wouldn’t have a significant meaning in the outcomes. What should also be improved is the accuracy in the adherence to the time the cubes should stay in the solution. The measurements are to be taken immediately after removing the cubes – it will prevent any undesired processes to start and change the results. A good idea to extent the experiment is to examine more cubes with more different sizes. This will give us an image on how does the correlation between SA:V and diffusion rate look in a full spectrum, does it stay in the same trend as this experiment has shown or not. 17.09.2014 13 Irena Sionek 2IB Bibliography: 1. Katarzyna Zaremba, „IB DIPLOMA PROGRAMME BIOLOGY SL AND HL STUDENT HANDBOOK” 2. „Writing a good biology lab report”, http://signatureibbiology.wikispaces.com/file/view/The+Perfect+Biology+Lab+Re port.doc, , Web, 11.09.2014 3. „How to write a good biology lab report”, http://www.ibsurvival.com/topic/13178how-to-write-a-good-biology-lab-report/, 21.02.2014, Web, 11.09.2014 4. „Diffusion”, http://en.wikipedia.org/wiki/Diffusion , 07.09.2014, Web, 12.09.2014 5. „Sodium hydroxide”, http://en.wikipedia.org/wiki/Sodium_hydroxide, 21.08.2014, Web, 13.09.2014 6. „What does stansard deviation show us about our data”, http://science.halleyhosting.com/sci/soph/inquiry/standdev2.htm, Web, 16.09.2014