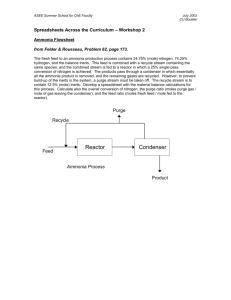
ASPEN Exercise 3 Ammonia synthesis process with a recycle stream for Aspen Plus 7.3.2 Flow diagram Gas purge 5% Recycle 95 % Heat Q kW Qchiller kW 25 oC 400 oC Reactor 0 oC Separator Pressure: 125 atm N2(g) + 3H2(g) 2NH3(g) (Equilibrium) Feed N2: 1000 lbmol/h H2: 2000 lbmol/h CH4: 8 lbmol/h Chiller Liquid NH3 Compute flow rate of NH3 product heat duty for the reactor Q, heat duty for chiller Qchiller Getting started Click on START All Programs AspenTech Process Modeling V7.3.2 Aspen Plus Aspen Plus v7.3.2 Click on “New”, “Blank Simulation”, and “Create”. A new document will open. Click the “Simulation” Select “Mixer” and drop one on the flowsheet. Select “Material Streams” and add two input streams and one outlet stream. As before, click on the flowsheet and then on the red input arrow to add the required input stream. Then click on the flowsheet and click where the red input arrow has turned to blue to add a second input. Then click the red output arrow and then on the flowsheet to add the output stream. Change the names of your streams to “Feed”, “Recycle”, and “FR-MIX”, and your mixer to “MIXER”. You’ll need to click on the arrow button in order to then right-click on your streams and rename them. Select the “Reactors” tab, then choose “REquil” for an equilibrium reactor, and drop it on your sheet. Rename your reactor “REACTOR”. Double-click “FR-MIX”. Your cursor becomes an arrow into a line. Click the red input arrow in the reactor. Zoom if necessary Now add a material output stream to the top of the reactor (“VAP-REAC”), and one to the bottom (“LIQ-REAC”). Also add an output heat stream (“QREAC”). We need a chiller. We use a heater block for that. Click the “Heat Exchangers” tab, then “Heater”, add one. You can rename this “Chiller”. Double-click the end of the “VAP-REAC” stream and connect it to the chiller. Add an output material stream (“CHILLED”) and output heat stream (“QCHILL”) to the chiller. Click the “Separators” tab, click “Flash2” for twocomponent flash (liquid/vapor), and drop it on your sheet. Name it “FLASH”. Connect “CHILLED” to the Flash input. Make two material output streams for the flash, “VAP-FL” (top) and “AMMONIA” (bottom). Click the “Mixers/Splitters” tab, then “FSplit”, and drop a tee splitter on your sheet; name it “TEE”. Connect “VAP-FL” to TEE’s input, and make an output material stream for TEE called “PURGE”. Now connect the beginning of the “RECYCLE” stream to an output of the TEE. The flowsheet is done. Click the “Next” button, then click OK to go to the data browser. Name your flowsheet “Example 3: Recycle” and select SI units. Click “Next”, then add Nitrogen, Hydrogen, Ammonia, and Methane. Click “Next”, and set “Base method” to be “PENG-ROB” for the PengRobinson equation of state. Click “Next”, and our interaction parameters are fine, so click “Next” and then “OK” to “Go to Simulation environment”. Set your feed stream specifications: T = 25 C, P = 125 atm, total flow = 3008 lbmol/hr. Set the feed components: Nitrogen = 1000, Hydrogen = 2000, Methane = 8. Click “Next” and set the chiller specs. T = 0 C, P = 0 atm (no pressure drop). Click “Next” and set the flash specs. T = 0 C, P = 0 (no pressure drop). Click “Next” and confirm the mixer spec is set to no pressure drop (P = 0). Click “Next” and set the reactor specs. T = 400 C, P = 125 atm Click “Next”, then “New”. Set the reactants as Nitrogen (-1 coefficient) and Hydrogen (-3 coefficient) Set the product as Ammonia (2 coefficient). Click “Next”, then “Next”, and set the PURGE value as 0.05. This is the split fraction. Note that everything else gets grayed out; you are done here. Click “Next”. It looks like you are done! Click OK. Watch the calculations. Note the warning. The warning is about an inconsistency, but it’s not too bad, and may be due to a big recycle stream. In the Data Browser, go to “Result Summary” . Click on “Streams”. Check the total amount in the recycle stream. Click on “Heat” tab to confirm the cooling duty Exercises: 1.Calculate the net production rate of ammonia from the process. What fraction of the ammonia produced in the reaction is lost in the purge stream? Why is it crucial to have a purge stream in this process? 2. Look at the heat flows in the process. What general idea could be considered to reduce the overall energy consumption of the process? 3. It is expensive to pressurize gases to high pressures. Determine how the production rate of ammonia would be affected by reducing the reactor pressure to 100, 75, or 50 atm. Can you explain the physical principle that causes this change? 4. It is also expensive to heat gases up to high temperatures. Determine how the production rate of ammonia would be affected by holding the reactor pressure at 125 atm but reducing the temperature to 350, 300, and 200 oC.