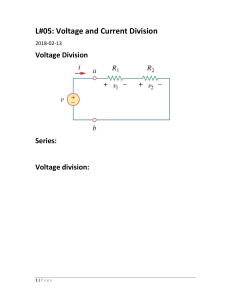
Chem 201B Dr. Baxley Conceptual Electrochemistry Questions modified from http://www.jce.divched.org/jcedlib/qbank/collection/conceptests/ For each question, write your answer and a brief explanation before looking at the answers (if you look at the answers before writing your answer and explanation, you won’t get as much out of this worksheet) For the first six questions, refer to the figure below. 1. Which equation below represents the correct chemical equation for the spontaneous halfreactions that will occur? a. Ni2+(in 1.00 M soln) + 2e– → Ni(s) and Ni(s) → Ni2+(in 1.00 x 10–3 M soln) + 2 e– b. Ni2+(in 1.00 x 10–3 M soln) + 2e– → Ni(s) and Ni(s) → Ni2+(in 1.00 M soln) + 2 e– c. no spontaneous reaction will occur 2. Will a voltage be measured for this cell? 3. Which correctly describes the electron flow? a. b. c. d. e– flow left to right through the salt bridge e– flow left to right through the wire and right to left through the salt bridge e– flow left to right through the wire e– flow right to left through the wire 4. To which cell (left or right) will the positively charged ions in the salt bridge flow? 5. If the pictured solutions are mixed and then divided into separate cells, will a voltage be measured? 6. What is the name of the type of voltaic cell shown in the picture? Chem 201B Dr. Baxley For the next two questions, refer to the following cell potentials. Ni2+(aq) + 2 e– → Ni(s) Eº = –0.23 V Cr3+(aq) + 3e– → Cr(s) Eº = –0.73 V 7. Which correctly represents reactions that may occur spontaneously? a. Ni(s) will react with Cr3+(aq) b. Cr(s) will react with Ni2+(aq) 8. Which of the following is the strongest oxidizing agent? a. b. c. d. Ni(s) Ni2+(aq) Cr(s) Cr2+(aq) A voltaic cell is created under standard conditions with the cell notation Cu(s) | Cu2+(aq) || Ag+(aq) | Ag(s) 9. If water is added to the Cu2+ cell, how will the voltage be affected? Answer this without looking at reduction potential values, just look at the cell notation. a. voltage will increase b. voltage will decrease c. voltage will not change 10. If ammonia is added to the Cu2+ cell to form an amine complex, how will the voltage be affected? a. voltage will increase b. voltage will decrease c. voltage will not change 11. If Cl– solution is added to the Ag+ cell to precipitate AgCl, how will the voltage be affected? a. voltage will increase b. voltage will decrease c. voltage will not change 12. As current passes, the voltage a. increases b. decreases c. does not change Chem 201B Dr. Baxley 13. As current passes, how will the concentrations of the ions change? a. b. c. d. e. f. [Cu2+] will increase by the same amount that [Ag+] decreases [Cu2+] will increase by twice the amount that [Ag+] decreases [Cu2+] will increase by half the amount that [Ag+] decreases [Cu2+] will decrease by the same amount that [Ag+] increases [Cu2+] will decrease by twice the amount that [Ag+] increases [Cu2+] will decrease by half the amount that [Ag+] increases If the reduction of mercury (I) in a voltaic cell is desired, the half reaction is: Hg22+(aq) + 2 e– → 2 Hg(l) Eº = 0.80 V 14. Which of the following reactions could be used as the anode (oxidation)? a. Sn4+(aq) + 2e– → Sn2+(aq) b. Br2(l) + 2e– → 2 Br–(aq) Eº = 0.15 V Eº = 1.07 V 15. For the voltaic cell created according to your answer above, what will be the anode material? 16. A concentration cell is created using an unknown metal and the metal cation. The concentration of metal cation in one half cell is 2.0 x 10–4 M and in the other cell it is 1.5 M. The cell voltage reads 0.0764 V. What is the charge on the metal cation? Chem 201B Dr. Baxley Answers 1. Answer a is correct. The high concentration Ni2+ would react to become a lower concentration and the low concentration Ni2+ would form as a product to increase its concentration. 2. Yes a voltage will be measured. 3. Answer c. The e– do not flow through the salt bridge, only through the wire. They will flow from the anode on the left to the cathode on the right. The direction of electron flow is determined by looking at the answer to #1. 4. The cations will flow through the salt bridge in the same directions as the e– from left to right. 5. No, a voltage will no longer be measured because the concentrations of Ni2+ on both sides will be equal. 6. This is a concentration cell. 7. Answer b, Cr(s) will react with Ni2+(aq). Since Ni2+ has a higher reduction potential, the Ni2+ ions are more likely to undergo reduction than the Cr3+ ions. 8. Answer b. Ni2+ ions will oxidize Cr(s) 9. Answer a. Since Cu is listed first in the cell notation, it is the anode, meaning that Cu(s) is oxidized to form Cu2+. Since Cu2+ is a product in the reaction, decreasing its concentration will increase the cell potential, which is measured in voltage. It helps to write the half-reactions and the overall reaction. The overall reaction is: Cu(s) + 2 Ag+(aq) → Cu2+(aq) + 2 Ag(s) 10. Answer a. Complexing the Cu2+ ions is another way to decrease its concentration. 11. Answer b. Ag+ is a reactant in the spontaneous reaction, so decreasing its concentration will lower the potential. 12. Answer b. As current passes, the reactant concentration will decrease and the product concentration will increase, which decreases the potential. 13. Answer c. Since Ag+ is a reactant, its concentration will decrease. Since the mol ratio is 2Ag2+/1Cu2+, the Ag+ changes at twice the rate that Cu2+ changes, so Cu2+ changes at ½ the rate of Ag+. 14. Answer a. The half reaction with the lower reduction potential will undergo oxidation in a spontaneous reaction. 15. Since the reactant and product in the half reaction occurring at the anode are both aqueous, an inactive electrode, such as platinum or graphite would be used. For the voltaic cell created according to your answer above, what will be the anode material? 16. n = 3. Use the Nernst equation and solve for n.