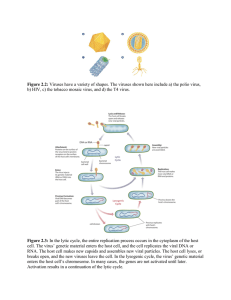
Microbial Viral Insecticides: Baculoviruses for Pest Control
advertisement

4 Microbial Viral Insecticides Aparna S. Kalawate Abstract Microbial viral insecticides are pathogens that attack insects and other arthropods. Baculoviruses (BV) are parasitically replicating microscopic elements. Baculoviruses are extremely small and are composed primarily of double-stranded DNA required for the virus to establish itself and reproduce. The genus Baculoviruses contains three subgroups of viral types: nuclear polyhedrosis viruses (NPVs), granulosis viruses (GVs) and nonoccluded viruses. NPVs and GVs differ in the number and structure of the protective protein coat and are both relatively large and complex in structure in comparison to many other types of viruses. While little information is available for viruses from the third subgroup, several aspects of the infectivity and mode of action of NPVs and GVs have been studied. The most common route of entry into an insect is by ingestion. The primary site of infection is the midgut cells by membrane function. However, two distinct mechanisms of virus uncoating occur among the baculovirus, that is, NPVs uncoat within the nucleus, whereas GVs uncoat within the nuclear pore complex. NPVs may pass through the intestinal epithelium immediately after ingestion, thereby establishing a systematic infection of the haemocoel prior to virus replication in the midgut cells. The GVs do not appear to pass through midgut cells as rapidly as NPVs, and the developmental cycle of GVs is longer than that of NPVs. The NPVs are mass produced in larval hosts grown on artificial diet or host plant. Usually third to fourth instar larvae of Helicoverpa armigera are infected with the viral food. The definitive phase of viral disease occurs over a period of 5–10 days. Once the complete infection of the virus in the larvae is completed, the larvae start ‘putrefying’ releasing billions of polyhedra. In commercial production, larvae are A.S. Kalawate (*) Entomology Section, Zoological Survey of India, Western Regional Centre, Sector-29, Vidya Nagar, Rawet Road, Akurdi, Pune-411044, Maharashtra, India e-mail: aparna_ent@yahoo.co.in K. Sahayaraj (ed.), Basic and Applied Aspects of Biopesticides, DOI 10.1007/978-81-322-1877-7_4, # Springer India 2014 47 48 A.S. Kalawate being harvested before purification to keep bacteria at a low level in the final product. After the larval production phase is complete, the larvae are collected and formulated. NPVs are ideal candidates for use where a single lepidopteran species is the major pest. NPVs are being used against H. armigera and Spodoptera litura on cotton, corn, sorghum, tomatoes and chrysanthemum. It is also being used against Anticarsia gemmatalis of soybean. One of the most important successes in commercial production and use of a GV is Cydia pomonella GV (CpGV) on apples and pears. Advantages in using microbial viruses are safety for humans and other nontarget organisms, reduction of pesticide residues, little or no development of resistance by the target organism, no secondary pest outbreak and no preharvest interval is required. Though there are many advantages, some disadvantages are also there, for example, host specificity is a double-edged sword; it is an advantage as well as a disadvantage. Moreover, long period of lethal infection is required, and the virus gets inactivated by environmental factors like ultraviolet light, extreme temperature, etc. In this chapter, an attempt has been made to cover the commercially available BVs for the control of agricultural pest particularly in India. The objective of this chapter was to briefly cover the aspects like importance of baculoviruses in pest control, history, genome and the products available in the Indian market. Keywords Baculovirus Viral insecticides and microbial control and entomopathogenic viruses 4.1 Introduction Biopesticides are certain types of pesticides derived from such natural materials as animals, plants, bacteria and certain minerals. The EPA separates biopesticides into three major classes based on the type of active ingredient used, namely, microbial, biochemical or plantincorporated protectants (GMOs). All aspects of the utilisation of microorganisms or their byproducts in the control of insect pest species are called microbial control. A virus is a small infectious agent that can replicate only inside the living cells of organisms. Viruses infect all types of organisms, from animals and plants to bacteria and insects. The study of viruses is known as virology, and the study of viruses causing diseases Biopesticides in insects is known as insect pathology. Inclusion viruses are submicroscopic, obligate, intracellular and pathogenic organisms. Seven families of viruses, namely, Baculoviridae, Reoviridae, Iridoviridae, Poxviridae, Parvoviridae, Picornaviridae and Rhabdoviridae, cause diseases in insects. But the viruses of families Baculoviridae and Reoviridae are the most important for their role as biopesticides because of their high virulence. 4.2 Classification Baculoviruses are different from vertebrate viruses and therefore safe to humans and other vertebrates. The classification of baculoviruses is represented in the following flow chart. 4 Viral Insecticides 49 Baculoviruses Baculoviridae (Viruses with nuclear inclusion bodies) Nuclear polyhedrosis viruses (NPV) Single-nucleopolyhedroviruses (SNPV) contain a single nucleocapsid per virion e.g. Trichoplusia ni SNPV (TnSNPV) 4.2.1 Baculoviruses The divisions viz., NPV and GV were recently challenged because the comparison of 29 fully sequenced baculoviral genomes indicated that virus phylogeny followed more closely the classification of the hosts than the virion morphological traits, but the traditional division into two genera is still widely used (Boguslaw Szewczyk et al. 2011). Baculoviruses have double-stranded genome with rod-shaped nucleocapsids. The infectious virus particles or virions are occluded in protein bodies called polyhedra (NPV) or granules (GV). NPV polyhedra are larger than the virions (usually 1–15 μm) and may contain many virions. The infection occurs after a susceptible host eats the polyhedra or granules, which are dissolved in the basic digestive gut juices. The virions are released when the protein matrices dissolve. The virions enter the nuclei of midgut cells and eventually infect many of the tissues and organs in the insect, primarily the fat body, epidermis and blood cells. The baculoviruses which are not occluded in polyhedra have recently been removed from the Baculoviridae group. The infection caused by baculoviruses is called ‘wilting disease’ because the larva becomes wilted and tissues of the host liquefy; infection of the epidermis causes the Reoviridae (Viruses with cytoplasmic inclusion bodies) Granulosis viruses (GV) multiplenucleoplyhedroviruses (MNPV) contain multiple nucleocapsid per virion e.g. Autographa californica MNPV (AcMNPV) host to appear to melt, releasing virus particles into the environment. Often just before death, the larvae climb to the highest part of the substrate and attach themselves by their prolegs. Baculoviruses are considered to be the most beneficial of the insect viruses to man, because of their utility in insect control, their specificity to the arthropods and their more recent use in fundamental biological studies using molecular techniques. Nevertheless, they also cause diseases in beneficial insects, and, therefore, the use in the environment as biological control agents requires an understanding of host range and the mechanisms that control host specificity (Miller 1997). 4.3 History The virus family Baculoviridae have been known for hundreds of years. The earliest record of baculovirus infection was in Chinese silkworms. Paralysis and subsequent liquification occurring in the larvae affected with baculoviruses were found in many ancient literature. It wasn’t until the early twentieth century that it was established that the virus particles were embedded in proteinaceous crystals of polyhedrin. This crystalline 50 matrix allows the virus to survive in the environment. It was at this stage that baculoviruses were suggested as a method of natural control of pest insect populations. In the 1930s and 1940s, rod-shaped virions were identified within the crystalline polyhedrin. During the same period, baculoviruses were observed to be an effective biological control agent of an insect pest. It was discovered that the spruce sawfly (accidentally introduced into North America) could be effectively controlled by the subsequent introduction of a baculovirus. The first baculovirus to be registered as a pesticide (in 1975) was a commercial failure. However, the use of a baculovirus as a pest control agent that was a nucleopolyhedrovirus used to control the Douglas-fir tussock moth in 1984 was a notable success. This has attracted many investigators to understand the molecular biology of the baculovirus and has led industrial interest in the commercialisation of it in the 1990s. Major achievements have been made in the field of baculovirology in the past two decades. These viruses now have a major role in the field of biomedical research as well as contributing to our understanding of the complex virus-host interactions. Baculoviruses are now being used for making them recombinant by utilising the genetic engineering for insect control. The first viral insecticide Elcar™ was introduced by Sandoz Inc. in 1975 (Ignoffo and Couch 1981). Elcar™ was a preparation of Heliothis zea NPV which is relatively a broadrange baculovirus and infects many species belonging to genera Helicoverpa and Heliothis. HzSNPV provided control of not only cotton bollworm but also of pests belonging to these genera attacking soybean, sorghum, maize, tomato and beans. In 1982, the production of this biopesticide was discontinued. The resistance to many chemical insecticides including pyrethroids revived the interest in HzSNPV, and the same virus was registered under the name Gemstar™. HzSNPV is a product of choice for biocontrol of Helicoverpa armigera (Mettenmeyer 2002). Countries with large areas of such crops like cotton, pigeon pea, tomato, pepper and maize, for example, India and A.S. Kalawate China, introduced special programmes for the reduction of this pest by biological means. In Central India, H. armigera in the past was usually removed by shaking pigeon pea plants until caterpillars fell from the plants onto cotton sheets. This technique is now used to obtain caterpillars which are fed on virus-infected seeds. Baculovirus preparations obtained in this way are used by farmers to prepare a bioinsecticide spray applied on pigeon pea fields. Another baculovirus, HaSNPV, is almost identical to HzSNPV. It was registered in China as a pesticide in 1993 (Zhang et al. 1995). It has been used for large-scale biopesticide production and has been extensively used on cotton fields (over 100,000 ha of cotton in the last decade). Broadspectrum biopesticide based on Ha NPV is also used in India (Srinivasa et al. 2008). The forests of temperate regions are very often attacked and defoliated by the larvae of Lepidoptera (most common pest species are Lymantria dispar, Lymantria monacha, Orgyia pseudotsugata and Panolis flammea) and some Hymenoptera species (mainly Neodiprion sertifer and Diprion pini). L. dispar MNPV formulations marketed under trade names Gypchek, Disparivirus and Virin-ENSH and O. pseudotsugata MNPV under trade names TM BioControl-1 and Virtuss (Reardon et al. 1996) are sometimes used for forest protection. Forest ecosystems tend to be more stable than agricultural systems, allowing for natural or applied baculoviruses to remain in the environment for long periods of time increasing the chance of natural epizootics by these agents. Caterpillars of moths belonging to Spodoptera genus are of primary concern for agricultural industry in many countries of the world. Two commercial preparations based on Spodoptera NPV have been available. These are SPOD-X™ containing Spodoptera exigua NPV to control insects on vegetable crops and Spodopterin™ containing Spodoptera littoralis NPV which is used to protect cotton, corn and tomatoes. About 20,000 ha of maize annually was controlled with Spodoptera frugiperda NPV in Brazil (Moscardi 1999), but at present it has not been used due to technical problems in 4 Viral Insecticides the virus production under laboratory conditions. The use of Spodoptera litura NPV has been tested on cabbage crops in India (Kumari and Singh 2009). Many other species belonging to the Noctuidae family are economically important pests of sugarcane, legume, rice and others. Autographa californica and Anagrapha falcifera NPVs were registered in the USA and were fieldtested at a limited scale. These two NPVs have relatively broad host spectrum and potentially can be used on a variety of crops infested with pests belonging to a number of genera, including Spodoptera and Helicoverpa. Granulovirus CpGV is the active component of a number of biopesticides used for the protection of apple and pear orchards against the codling moth Cydia pomonella. Some of the trademarks of CpGV-based products are: Granusal™ in Germany, Carpovirusine™ in France, Madex™ and Granupom™ in Switzerland and Virin-CyAP in Russia. Annually up to 250,000 ha of orchards has been protected with Madex™ in different European countries (Vincent et al. 2007). Considering application of all trade names of the CpGV, this may be the most important worldwide viral insecticide currently applied in terms of treated area. Other important viruses that are currently employed to control insects include the tea tortricids Adoxophyes honmai and Homona magnanima granuloviruses (GV) in Japan. The area sprayed with GVs comprised 5,850 ha in Kagoshima in 1995, equivalent to 80 % of all the tea fields in the prefecture (Nishi and Nonaka 1996). The GVs of H. magnanima and A. honmai were registered in 2003; however, the use of GVs has recently declined. One reason for the reduction in use of GVs in Japanese tea fields is the changing pattern of occurrence of other pests. Mulberry scale, for example, has been increasing recently, and chemical treatment is required to control this insect and at the same time GVs are sprayed. The spray also kills H. magnanima and A. honmai. Furthermore, GVs have been applied in Kagoshima for more than 10 years, and the populations of H. magnanima and A. honmai have been reduced (Nakamura 2003). In China, 12 baculoviruses have been authorised as 51 commercial insecticides (Sun and Peng 2007), including H. armigera NPV (the most widely used virus in China for cotton, pepper and tobacco protection), S. litura NPV (vegetables), S. exigua NPV (vegetables), Buzura suppressaria NPV (tea), Pieris rapae GV and Plutella xylostella GV (vegetables). The use of baculoviruses in China is the greatest worldwide, regarding the number of viruses being registered for insect control. Sun and Peng (2007) also reported a cypovirus (CPV) produced in China for the control of Dendrolimus punctatus, an insect pest of pine forests. The well-known success of employing baculovirus as a biopesticide is the case of Anticarsia gemmatalis nucleopolyhedrovirus (AgMNPV) used to control the velvet bean caterpillar in soybean (Moscardi 1999). This programme was implemented in Brazil in the early 1980s and came up to over 2,000,000 ha of soybean treated annually with the virus. Recently this number dropped down, mainly due to new emerging pests in the soybean complex. The use of AgMNPV in Brazil brought about many economical, ecological and social benefits. At the soybean grower level, the financial savings from the use of the virus may reach up to ca. U$ 7/ha/ season, including product cost and application cost. The annual savings at the grower level, in the total area sprayed with the virus, was over US$ 11,000.000. Since the beginning of the programme, more than 17 million litres of chemical insecticides was not sprayed in the environment. The protection of soybean fields in Brazil has proven that baculoviral control agents can be effectively produced on a large scale and they may be an alternative to broad spectrum chemical insecticides. On the basis of this spectacular success of a baculovirus pesticide, it is needless to say that the advantages of biopesticides over chemical pesticides are numerous. Safety for humans and nontarget organisms, preservation of biodiversity in the environment and reduction of toxic residues in agricultural end products are just the examples of potential benefits. However, the cost of biopesticide production has been usually higher than the cost of conventional pesticides (Boguslaw Szewczyk et al. 2011). Genomic variability has been described for many wild-type viruses including A. californica 52 A.S. Kalawate MNPV, S. frugiperda MNPV, S. litura MNPV, P. flammea MNPV and Mamestra configurata NPV. Genotypic variants can be recognised by the presence of submolar fragments in the electrophoretic patterns of restriction endonuclease digestion products of a viral genome. Genotypic variation in baculovirus genomes can include point mutations, both small and large deletions and insertions (Krell 1996). Though mutations can occur in any place of the genome, the presence of some hot spots was observed for certain genomic alterations such as insertions due to transposable elements or deletions in the hypervariable DA26 gene region (Kamita et al. 2003). AgMNPV genomic variability has been also carefully studied because the selection pressure due to the application of AgMNPV in the field during subsequent years could lead to alterations in virus stability. The method of choice was the technique of restriction endonuclease analysis. Viral DNAs were initially purified from diseased larvae collected during several crop seasons and compared to AgMNPV-79, a wild-type virus that was used originally and subsequently in this programme (Souza et al. 2001). These results indicated that the virus maintains considerable stability, even with the existence of some genetic changes shown in the DNA restriction profiles. It has been also observed that the virus retains its virulence to the host insect throughout the years of its application. 4.4 Future Use of Baculovirus Pesticides Large-scale application of AgMNPV in Brazil has proven that the baculovirus protection can be done at relatively low cost. It is very likely that the growing awareness of the benefits of the environment-friendly pesticides will result in the re-evaluation of the prospects for biological protection with baculovirus preparations. However, until today, baculovirus insecticides have not met their full potential to control pest insects worldwide. The development of recombinant baculovirus was efficiently completed by researchers in several countries, but the in vitro commercial technology still lags, due to technical problems. Future development of baculovirus pesticides will probably depend on the attitude towards genetically modified organisms. In countries where use of genetically modified organisms is restricted, only naturally occurring baculoviruses will be used for protection of crops. In this case the improvements will be at the level of diagnostics of infection, development of the in vitro cultures and changes in the formulations of the biopesticide. In countries which favour the introduction of genetically modified organisms, the improvements will be achieved by introduction of exogenous genes into baculovirus genome, thus greatly enhancing the killing activity of bioinsecticide formulations. Reliable assays for the progress of infection with baculovirus are necessary because the major problem in using biopesticide for crop protection is their slow action and lack of morphological changes in larvae in first stages of baculovirus propagation. The lack of such assays may incline agricultural services to use subsequent chemical means of protection which, from the ecological point of view, may be redundant. Fast and sensitive methods in diagnostics based on baculovirus genome detection will probably play a predominant role in future. For strictly quantitative assays, real-time PCR is the best method. The in vitro production is still a strong requirement on a commercial perspective of baculoviruses use as insecticides. However, the accumulation of genotypic variations by serial passage in cell culture prevents its large-scale production. One of the most important effects of the viral passage is the change from the parental, many polyhedra per cell (MP) phenotype, to the few polyhedra per cell (FP) phenotype. The major problem of the passage effect is the reduced occlusion and loss of virulence of the occluded virus (Krell 1996). Frequent mutations have been identified within a specific region in the few polyhedra (FP) mutants that contains the 25 k fp locus (Harrison and Summers 1995; Lua et al. 2002). This gene encodes a 25 kDa protein that is essential for virion occlusion and polyhedron formation. Another type of mutants generated during serial passage of baculovirus is the formation of defective interfering particles (DIPs). 4 Viral Insecticides These mutants have lost the ability to be replicated in the host cell without the aid of a helper virus, and large sizes of their genome are usually deleted (Pijlman et al. 2001). These particles replicate faster because they are smaller and inhibit the replication of a standard virus. The challenge to make in vitro commercial production of baculoviruses a viable initiative depends on the development of new techniques to sustain MP production through passages in cell cultures from small flasks to large-scale commercial fermentors. The stability of baculoviruses is influenced by temperature, pH, humidity and the presence of additives, but ultraviolet light is probably the most detrimental factor to viral survival. Under field conditions, little activity is left when the virus is not shaded by plant canopy; therefore, much effort has been devoted to the development of UV protectants (Shapiro and Dougherty 1994; Zou and Young 1994; Morales et al. 2001). The best results were obtained for stilbene fluorescent brighteners which are marketed under many trade names (e.g. Phorwite AR, Blankophor and others). Future developments in the formulations of brighteners may lead to the reduction of cost of baculovirus production. Inactivation of baculoviruses may be also caused by plant metabolites such as peroxidases which generate free radicals (Hoover et al. 1998). The inactivation can be reduced by addition of free radical scavengers such as mannitol or enzyme superoxide dismutase to baculovirus preparations (Zhou et al. 2004). The inactivation of Ha NPV was found to be reduced when it was sprayed in combination with adjuvants like Leucaena leaf extract, eucalyptus leaf extract and Ranipal in the morning and evening (Kalawate and Nachane 2006). The activity of baculoviruses against their natural hosts may be enhanced by introduction of insect-specific toxins or by interference with insect physiology (Bonning and Hammock 1996; Inceoglu et al. 2001). Baculovirus genome modifications by introduction of exogenous toxin genes were extensively studied in many laboratories. Most of the research was devoted to the studies of arthropod toxin genes isolated from the scorpion or spiders (Bonning and 53 Hammock 1996; Inceoglu et al. 2007). The most potent insect-specific toxin gene used for construction of baculovirus recombinants was the gene coding for a toxin from scorpion Androctonus australis. The feeding damage caused by larvae infected with this modified baculovirus was reduced by about 60 % in comparison to a wild-type baculovirus (Inceoglu et al. 2001). Toxin genes isolated from other scorpions, for example, Leiurus quinquestriatus hebraeus (Froy et al. 2000), straw itch mite Pyemotes tritici (Burden et al. 2000), ants (Szolajska et al. 2004) or spiders (Hughes et al. 1997) have been intensively studied as potential enhancers of baculovirus activity. Arthropod toxins usually attack insect sodium channels producing final effect similar to the chemical insecticides of the pyrethroid group. However, the specific target in sodium channels is different, so there is a potential possibility to produce synergistic effect by biopesticide/chemical pesticide application (McCutchen et al. 1997). Baculovirus recombinants that produced occlusion bodies incorporating Bacillus thuringiensis toxin were constructed by making a fusion protein consisting of polyhedron and Bt toxin (Chang et al. 2003). The pathogenicity of the recombinant was remarkably increased compared to wild-type virus. These studies proved that it is possible to construct a biopesticide which combines the advantages of the virus and the bacterial toxin. The changes to host physiology were done by introducing genes coding for some insect hormones or hormone-modifying enzymes into baculovirus genome or by deletion of the baculovirus-encoded ecdysteroid glucosyltransferase (egt) gene. The former approach was employed by cloning juvenile hormone esterase gene into baculovirus genome which overexpressed decreases the concentration of the juvenile hormone which is a signal for a caterpillar to stop feeding and pupate. This line of research is being pursued in some laboratories (Hammock et al. 1990; Inceoglu et al. 2001). The deletion of the baculovirus-encoded egt gene was used first by O’Reilly and Miller (1991). The product of the egt gene interacts with larval moulting and indirectly increases the time of feeding of 54 A.S. Kalawate infected caterpillars. The egt deletion from baculovirus genome resulted in 30 % faster killing of caterpillars. Another advantage of this genomic modification is the fact that the egt gene is not essential for viral replication and can be replaced with an exogenous gene, the product of which may enhance the insecticidal activity of the recombinant virus (Sun et al. 2004). In the future, genetically modified baculoviruses will contribute to the expansion of baculovirus use worldwide, as these GMOs are considered safe through extensive research conducted over many years. The scientific data indicate that baculoviruses pose no hazard to other animals than their hosts, and this was documented by a number of studies from different laboratories. Recombinant baculoviruses were not pathogenic to bees and all vertebrate species (Sun et al. 2004) as well as to the natural enemies of larvae such as parasitoids and predators (Boughton et al. 2003). However, in spite of this sound evidence, preliminary field trials of genetically modified baculoviruses raised massive public protests which put on hold further trials for a long time. The slow progress in application of genetically modified baculoviruses as pesticides may be in part due to the choice of toxin genes used for modifications of the baculovirus genome which were isolated from highly dangerous invertebrates. Taking into account the origin of these social conflicts, the choice of toxin genes used for genome modifications should be restricted to genes coding for ecologically natural insect toxins, for example, the genes coding for toxic polypeptides of parasitoid wasps occurring in regions infested by a particular pest. The more rational approach is also needed in the social perception of dangers associated with genetically modified baculoviruses by educating the public on risks and benefits of recombinant baculovirus pesticides (Boguslaw Szewczyk et al. 2011). 4.5 Genome lo-2 hr 6 -e5 1 ie- odv 53 e- hr 4 p7 m G B C H J od v-e 2 Orf 5 94 Orf93 p le 6.9 f-5 as e T S Q M D fp f-9 lic H 1 f7 Or -3 lef f73 Or -2 lap 75 Orf Orf76 he G B W BamHI EcoR I Hind III B 39 Orf -6 lef A le A A UV R E G EppoMNPV 120 kbp D I H b N K 4 1 1 f r a N O O 9 0 1 YZ Orf f108 7 r P O rf10 -4 O lap 7 K p8 04 2 1 0 f Or rf1 O Fig. 4.1 Eppo MNPV genome map (Kalmakoff and Ward 2007) X J J L F K F I lef-4 Orf122 Orf120 hr C M L E D C eat gp-64 I F 0 rep E Q p1 1629 polh Orf4 Orf5 lef-2 Orf1 lef- 1 egt 1 Or O f15 Or rf16 O f17 O rf2 rf2 0 1 Circular and double-stranded DNA genome has been found in baculoviruses. The genome size of these viruses ranges in size from 80 to 180 kbp. Of the fully sequenced baculovirus genomes, the number of open reading frames (ORFs) ranges from approximately 120 to 160 (Fig. 4.1). In addition to the genes encoded in the genome, there are also a number of small repeated sequences known Orf46 Orf48 gta Orf49 odv-e 66 4 Viral Insecticides 55 Fig. 4.2 Baculovirus particles or polyhedra (a); cross section of a polyhedron (b) and diagram of polyhedron cross section (c); electron micrographs (a and b) by Jean Adams, graphic # by V. D’Amico) as homologous regions (hrs) interspersed in the genome. These regions have been shown to enhance early gene transcription and also to act as origins of replication. Many of the genes in a baculovirus genome have overlapping ends allowing a large number of genes to be encoded in a smaller amount of DNA (Kalmakoff and Ward 2007). Baculoviruses have gained great attention in molecular biology laboratories because they are very versatile genetic engineering tools (Van Oers 2006). Current knowledge about the biology of AcMNPV is to a large extent a consequence of the developments of baculovirus-based expression vectors. Baculovirus system of expression of foreign genes has many advantages over other systems because high level of foreign gene expression is usually achieved compared to other eukaryotic expression systems (Boguslaw Szewczyk et al. 2011). Baculovirus genome can accommodate large pieces (up to 50 kbp) of foreign DNA, so it is possible to express more than one foreign gene. Additionally, the insertion of specific signal sequences in front of a foreign gene leads very often to the export of the gene product outside of the infected cell (Boguslaw Szewczyk et al. 2011). 4.6 Structure A distinctive rod-shaped nucleocapsid which is 30–60 nm in diameter and 250–300 nm in length is present in baculoviruses. GVs are occluded with dimensions of about 0.3 0.5 μm. The occluded NPVs are polyhedral in shape, and the size of it is approximately 0.15–15 mm. The occluded form of both the baculoviruses (GVs and NPVs) can clearly be seen using a light microscope. The occlusion-derived virus (ODV) is produced in the later stages of viral infection and is enclosed in a proteinaceous occlusion body. The spread of the virus from insect to insect is horizontal, and the virus persists for long periods in the environment. Baculoviruses also have a second morphology. This second form of the virus is found within an infected insect. This form is known as budded virus (BV). BVs generally contain a single nucleocapsid and are enclosed in an envelope obtained as the nucleocapsids bud out through the cell wall. Prior to the budding of the virus, the cell wall is modified by the addition of the viral protein GP64. This protein has been shown to be required for effective spread of the virus within the host (Fig. 4.2). 56 4.7 A.S. Kalawate Life Cycle 4.8 The infection of baculovirus starts with the ingestion of the virus-infected material by the insect larvae (Fig. 4.3). Death of the larvae occurs in 3–8 days depending on the larval species and instars (Table 4.1). The life cycle of baculovirus involves two forms of virus, that is, occlusion-derived virus (ODV) and budded virus (BV). The ODV is responsible for the primary infection of the host and is present in a protein matrix of polyhedron or granulin. The BV is released during the secondary infection from the host cell (Fig. 4.4). When a susceptible insect feeds on the virus-contaminated plants, the initial infection occurs. The protein encapsulating the baculovirus DNA dissolves in the alkaline midgut of the larvae releasing ODV. These ODVs then fused with the columnar epithelial cell membrane of the midgut and are taken into the cell in endosomes. Nucleocapsids are then transported to nucleus. Baculovirus DNA is then replicated in the cell nucleus until the rupture of midgut cells takes place. The development of BV occurs and the secondary infection starts. The infection spreads throughout the body in the haemolymph and infects the cells of haemocoel, fat bodies, trachea and hypodermis of the larvae. At this stage, the larvae stop feeding and die eventually (Fig. 4.4). There are different types of proteins present in baculoviruses which are required to carry the infection in the host. The different types of proteins and their functions are presented in Table 4.2. Relative Effectiveness It is widely acknowledged that baculoviruses can be as effective as chemical pesticides in controlling specific insect pests. However, the expense of treating a hectare of land with a baculovirus product invariably costs more than an equally efficacious chemical treatment. This difference in price is due primarily to the labour-intensive nature of baculovirus production. Some viruses can be produced in vitro (within cell cultures in the laboratory, not requiring whole, living insects). These are less expensive than those that can only be produced in vivo, that is, inside of living insects. The cost of rearing live hosts adds greatly to the final cost of the product. It is to be hoped that insect cell culture systems currently being developed for other uses may ultimately make viral pesticides more cost-effective. 4.8.1 Appearance The insects that are killed with baculovirus have a characteristic shiny-oily appearance and are often seen hanging limply from vegetation. They are extremely fragile to the touch, rupturing to release fluid filled with infective virus particles. This tendency to remain attached to foliage and then rupture is an important aspect of the virus life cycle. As discussed above, infection of other insects will only occur if they Table 4.1 Phases of baculovirus infection Phase(s) Early (0–6 h postinfection) Late (6–24 h postinfection) Very late (or occlusion) (18–24 to 72 h postinfection) Description Expression of genes involved in the replication of the virus and manipulation of the host. Delayed early genes often require the presence of viral transregulators (e.g. IE-0, IE-1, PE38) for efficient transcription Transition from early to late is characterised by shutdown of the host cell DNA replication and protein synthesis. Nucleocapsids are produced. Budded virus is produced and disseminates the virus throughout the host Advanced stage of virus infection. Virions become occluded in the protein polyhedrin. Viral proteases liquefy the host and degrade the chitinous exoskeleton. Occluded progeny virus is disseminated onto surrounding material for horizontal spread. The extensive lysis of cells frequently causes the host insect to literally melt, and this is called ‘wilting disease’ 4 Viral Insecticides 57 Fig. 4.3 The mode of action of baculovirus (Ramon Georgis 1996) Fig. 4.4 General overview of the replication cycle of baculoviruses (Kalmakoff and Ward 2007) ation Replic n tio Early ca pli Re Budded virus Late Polyhedra dissolve Fusion to midgut cell Dissemination Budded virus Dissemination eat foliage that has been contaminated by virus-killed larvae. It is interesting to note that most baculoviruses, unlike many other viruses, can be seen with a light microscope. The polyhedra of many viruses look like clear, irregular crystals of salt or sand when viewed at 400 or 1,000. The fluid inside a dead insect is composed largely of virus polyhedra – many billions are produced inside of one cadaver. Occluded virus Lysis 4.8.1.1 Habitat Baculoviruses can be found wherever insects exist. Because rain and wind readily carry baculoviruses from place to place, it is likely that every piece of land and body of water contains some virus particles. It is widely accepted by researchers that most produce currently on the shelves is ‘contaminated’ by baculovirus particles (Heimpel et al. 1973). In fact, the pervasiveness of 58 A.S. Kalawate Table 4.2 The important role of proteins in baculovirus infection Protein Polyhedrin/granulin GP64/F-protein EGT P35, IAP-1, IAP-2, IAP-3, IAP-4 DNApol IE-0, IE-1, IE-2, PE38 LEFs (at least 18) P6.9 Ubiquitin Cathepsin and chitinase Function Hyper-expressed protein which produces the crystalline matrix of the occlusion bodies. Provides protection from environmental damage Present on budded virus only; envelope fusion protein required for efficient entry of the budded virus into cells Enzyme for inactivating the host moulting hormones, ecdysteroids Inhibitors of apoptosis – prevent or delay cells from undergoing programmed cell death Viral DNA polymerase – required to replicate the viral genome Transactivators produced early in the replication cycle. Regulate the activity of other genes especially early in the replication cycle Late expression factors – required for the expression of late genes. Some also act to downregulate host cell activities Dephosphorylation of this protein is required for DNA packaging. Phosphorylation on viral entry into the cell leads to the DNA unwinding Has similarity to eukaryotic ubiquitin. May act by blocking the degradation of selected proteins during viral infection Possible role in damaging peritrophic membrane to aid initial infection. Required for liquefaction of the host and hence dissemination of the progeny virus baculovirus particles, along with the results of tests performed in conjunction with registration, may be considered both indirect and direct evidence for the safety of these agents. 4.8.1.2 Baculovirus Hosts Over the years, baculoviruses have been reported from a variety of different species of invertebrates. However, the only well-documented hosts belong to the order of Diptera, Hymenoptera and Lepidoptera. In some of the literature, it has been reported that occluded virions resemble NPVs in a caddis fly (Trichoptera) (Hall and Hazard 1973) and a shrimp species (Couch 1974). An occluded baculovirus-like virus was also reported for a thysanuran, but it did not appear to affect its host and transmission studies failed (Larsson 1984). Baculoviruses have also been reported from Orthoptera (Henry and Jutila 1966), but later these were classified as pox viruses, and from Coleoptera, but these are normally not occluded and were later placed in an unassigned category. Reports of infection of other insects, for example, a coleopteran (Ryel and Cline 1970), could not be confirmed. However, the infection occurred under laboratory conditions, where neuropterans were fed on Lepidoptera that had died of an NPV infection. Consequently, the neuropterans were likely heavily contaminated from their food source, and although they appeared to die of an NPV infection, they were probably exposed to an unusually high virus dose. Naturally infected Neuroptera have not been documented. 4.9 Pesticide Compatibility Viruses particles per se are generally unaffected by pesticides, although some chlorine compounds should be expected to damage or destroy viruses if applied at the same time. Baculovirus efficacy, however, can be altered in many ways by the effects of chemical pesticides on the host insect. A review by Jacques and Morris (1981) showed that of 10 pesticide-virus combinations, 9 resulted in an additive effect on insect mortality. However, some of the pesticides included in that review have since been banned. More work is needed to explore the effectiveness of insecticide ‘cocktails’ consisting of environmentally friendly chemical agents and baculoviruses in India. 4.10 Recombinant Baculoviruses Recombinant baculoviruses are usually constructed in two steps. Initially, a heterologous gene is introduced into a baculovirus transfer 4 Viral Insecticides vector. It consists of a bacterial replicon of a multicopy plasmid, a selection marker gene, promoter and terminator regions along with flanking baculovirus sequences from a nonessential locus and a multiple cloning site (or a single unique restriction site) downstream from a viral promoter. Most often the promoters and the flanking DNA originate from one of the late genes: polyhedrin or p10 gene. The latter is another viral gene coding for a protein which is produced in large quantities late in the infection. It is the main component of the fibrillar structures which accumulate in the nucleus and in the cytoplasm of infected cells. For some purposes, weaker early promoters, such as basic protein promoter (p6.9), may be preferred (Boguslaw Szewczyk et al. 2011). Around 400 insect cell lines are known which potentially can be used for in vitro propagation of baculoviruses. Only a few of them support the growth of AcMNPV. These lines were obtained from two parental organisms: Spodoptera frugiperda and Trichoplusia ni (Lepidoptera: Noctuidae). The most widely used line is Sf9 which grows well in suspension. BTI-Tn5B1-4 derived from T. ni, known as High Five cells, has been also largely used for viral growth (Granados et al. 1994). Cell lines which can be used for the propagation of Lymantria dispar nucleopolyhedrovirus (LdMNPV), Heliothis zea nucleopolyhedrovirus (HzSNPV), Bombyx mori nucleopolyhedrovirus (BmNPV), Anticarsia gemmatalis nucleopolyhedrovirus (AgMNPV) and a few other baculoviruses are also currently available (Boguslaw Szewczyk et al. 2011). 4.11 Baculovirus Production Technology At present, commercial production of baculoviruses has been carried out only in vivo, either by applying the virus against the host insect in the field and collecting diseased or dead larvae or by producing the target insect in the laboratory on an artificial diet. The latter is the most commonly used method for producing baculoviruses in many countries, but both methods have been 59 used successfully for the commercial production of the Anticarsia gemmatalis baculovirus (AgMNPV) in Brazil (Moscardi 1999, 2007). For some insects, there are no available artificial diets, and, therefore, the commercial production of baculoviruses of these baculovirus biopesticides 27 insects is generally too difficult or impossible under laboratory conditions. In such cases, field production of baculovirus stocks may be sometimes a method of choice, also from financial point of view (Moscardi 1999). In laboratory culture, the production of occlusion-derived virions (ODV) is not necessary for the survival of the virus. The budded virus (BV) particle is the form used for cell-to-cell transmission in cell culture. The main protein of the BV particle is the GP64 (Blissard 1996), essential for virus budding and responsible for entrance of the virus into the next host cell. Various culture conditions are known to influence infection of lepidopteran cells by baculoviruses and include temperature, pH, dissolved oxygen concentration, osmolality and nutrient composition of the culture medium. The investigation on factors associated with loss of genetic stability and the use of new strategies such as isolation of more stable variants, as well as the reduction of costs of cell culture medium components, are important requirements for process optimisation of in vitro baculovirus production. The requirements for productive insect cell lines (Jem et al. 1997) and for highly productive culture media (Chakraborty et al. 1999) are other challenges for in vitro production of baculovirus. Many cell lines are available for production purposes and are derived from various sources, thus exhibiting a wide variety of growth and production characteristics. Careful screening or formulation of media must be performed for a particular virus isolate cell line combination, as different media can greatly affect polyhedra yields (Pedrini et al. 2006). Recently, a new strategy for in vitro production was proposed based on many polyhedra (MP) variants. These are clones selected using the plaque assay technique after several passages of the virus in cell culture. MPs maintain the wild-type features such as formation of many polyhedra in the cell 60 A.S. Kalawate nucleus and budded virus high titre (Slavicek et al. 2001; Pedrini et al. 2005) which allow them, in principle, to compete with the population of few polyhedra mutants accumulated in cell culture. 4.12 Baculoviruses: Indian Scenario Biopesticides fall under the Insecticide Act (1968) under which any microbial organism manufactured or sold for pest and disease control should be registered with the Central Insecticides Board (CIB) of the Ministry of Agriculture. The national agricultural research system, comprising of the many ICAR institutes as well as state agricultural universities, plays a leading role in promoting biopesticides. The Project Directorate of Biological Control is involved in testing the quality of biopesticides and training the officers of the state department of agriculture in quality control protocols. The National Centre for IPM routinely incorporates the use of biopesticides in its IPM validation programmes and demonstrations, as do the IPM centres of the Directorate of Plant Protection, Quarantine and Storage. Commodity research boards have also played a role in researching and developing biopesticides for pest control in key crops such as cotton, coffee, tea and cardamom. Other biopesticides currently under development include Hyblaea puera NPV for controlling teak defoliator (Biji et al. 2006) and Amsacta albistriga NPV for controlling this pest on groundnuts. Baculovirus group has a very narrow host range and generally infests the larvae of crop pests. The research aimed at insect pest control is, therefore, confined to nuclear polyhedrosis viruses (NPVs) and granular viruses (GVs). In India, extensive research has been conducted on the use of NPVs for tackling two major pests, namely, Spodoptera litura and Helicoverpa armigera. Nuclear polyhedrosis viruses like Ha NPV and Sl NPV are increasingly being used as alternatives to chemicals. These viruses have distinct advantages over other methods of pest control. NPVs are virulent pathogens of insect characterised by the polyhedral occlusion bodies (POB). These viruses are highly specific and do not affect beneficial insects like parasitoids and predators and are safe to fish, birds, animals and man. Considering the usefulness of NPVs, there has been a growing demand amongst the farmers for these bioagents. The Government of India allocates funds for IPM programmes for all major crops, but these funds are mainly implemented at the state government level, through programmes promoting the use of biopesticides to farmers. Major national research programmes such as the National Agricultural Technology Project (2000–2006) and the current National Agricultural Innovation Project also contain important biopesticide research and development components. At the state level, 50 % of the plant protection budget is allocated to ecofriendly agriculture (Singhal 2004) to cover both the training of farmers and the procurement of biopesticides for distribution. A website on ‘biocontrol strategies for eco-friendly pest management’ has been launched recently by the Department of Biotechnology (DBT). The DBT has had a substantial funding programme for the research and development of microbial pesticides since 1989, with over 200 projects funded (Wahab 2004). This encourages the development of new technology and academic industrial links. The DBT also provides financial support for the generation of toxicological data to promote registration of microbials; data generation has been completed for almost all the currently registered biopesticides. The state governments play the main role in implementing IPM. Their IPM programmes for purchasing and distributing biopesticides to farmers have been vital to creating a market for and encouraging private commercial production of microbial pesticides. States such as Tamil Nadu, Gujarat, Andhra Pradesh and Maharashtra have been particularly active in promoting microbial pesticide use. The State Universities of Agriculture have played important roles in biopesticide research and in a few cases are also producing biopesticides themselves and are advising companies in production. The State Agricultural Universities and other stakeholder agencies, through the Agricultural Sciences Centre (Krishi Vigyan Kendra), are encouraged to take up initiatives to promote local production of microbial pesticides. Indian companies have 4 Viral Insecticides formed a biopesticide supplier’s association, the All India Biotech Association, to coordinate the commercial sector’s voice in developing government policy. Other organisations actively promoting biopesticides include nongovernmental organisations (NGOs) such as the M.S. Swaminathan Research Foundation and international research centres based in India such as the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) and the International Rice Research Institute. 4.12.1 Major Equipment Required The major equipments like centrifuge, laminar flow, magnetic shaker, microscopes, autoclave, coolers, refrigerators, incubator, distillation units, etc., are required in addition to glassware, plastic trays, basins and iron racks for mass production of Ha NPV and Sl NPV. Spodoptera litura (tobacco caterpillar): Spodoptera litura commonly known as tobacco caterpillar is a polyphagous pest. It is a serious pest of tobacco nurseries and also a sporadic pest of cauliflower, cabbage, castor, cotton, groundnut, potato and lucerne. It causes serious crop losses. Sl NPV: The virus is specific and infects only tobacco caterpillar. NPV can be successfully multiplied on tobacco caterpillar, and the viral extraction can be applied in the field to control the caterpillar. For continuous production of Sl NPV, it is necessary to rear tobacco caterpillar larvae continuously in a lab condition. Gram pod borer (Helicoverpa armigera): It is widely distributed in India and infests/damages a variety of cultivated and wild plants throughout its distribution range. It is a serious pest on commercial crop like cotton; pulses like red gram and Bengal gram; vegetables like tomato, bhendi and dolichos bean; oilseeds like sunflower, soybean and safflower; and cereals like sorghum and maize. Ha NPV: Ha NPV is a highly infective microbial biopesticide which can be used to control gram borer. It is being made from naturally diseased or under laboratory conditions artificially infected larvae of gram borer. 61 4.12.2 Mass Production of Ha NPV and Sl NPV The mass production of Ha NPV and Sl NPV involves 3 steps: (1) rearing of adult gram pod borer and tobacco caterpillar for mass production of eggs, (2) rearing of larvae of the above species either on the host plants like chickpea and castor under seminatural condition or on the synthetic diet in the laboratory conditions. In the model only the latter is considered for large-scale commercial production of NPV and (3) inoculation of Ha NPV and Sl NPV into the larvae of gram pod borer and tobacco caterpillar, respectively, for mass multiplication of viruses and extraction of polyhedral occlusion bodies (POBs) from the diseased larvae, which are used as biopesticide on the crop plants. 4.12.2.1 Details of Mass Production Diet preparation: The larvae of gram pod borer and tobacco caterpillar can be multiplied by using chickpea-based semisynthetic diet. The composition of the diet for rearing larvae is as follows: Item ‘A’ fraction: Chickpea (Kabuli chana) flour Methyl para-hydroxy benzoate Sorbic acid Streptomycin sulphate 10 % formaldehyde solution ‘B’ fraction: Agar-agar ‘C’ fraction: Ascorbic acid Yeast tablets Multivitaplex Vitamin E Distilled water Quantity 105.00 g 2.00 g 1.00 g 0.25 g 2.00 ml 12.75 g 3.25 g 25 tablets 2 capsules 2 capsules 780.00 ml Three hundred ninety ml of water is mixed with fraction ‘A’ of the diet in the blender which is run for 2 min. Fractions ‘A’ and ‘C’ are mixed, and the blender is run again for 1 min. Fraction ‘B’ is boiled in the remaining 390 ml water, added to the mixture of A and B, and the blender is run for a minute. Formaldehyde solution is added at the end, and the blender is again run for a minute. 4.12.2.2 Mass Production of Eggs Tobacco caterpillar: The culture of tobacco caterpillar is initiated by collecting eggs from the fields 62 of castor, cauliflower, lucerne, tobacco, etc. These field-collected eggs are reared in isolation to eliminate the emerging parasitoids and diseases, if any. The culture can also be established by collecting the gravid females with the help of light traps. Once the pure culture is established, the mass production is commenced under laboratory conditions after the first generation established. Pairs of newly emerged moths of tobacco caterpillar are placed in well-ventilated plastic containers. The inner wall of the containers is lined with paper to enable the adults to lay eggs. The bottom of the container is lined with sponge covered over by blotting paper. The moths are provided with 50 % honey solution and water on two cottons swabs placed in small plastic cups. The eggs which are generally laid in batches on the paper are cut out. Freshly laid egg masses are sterilised by dipping in 10 % formalin for 30 min, washed in running water for 30 min, dried on blotting paper and kept for hatching in sterilised glass vials. The freshly laid eggs can also be surface sterilised in 0.05 % solution of sodium hypochlorite for 5 min. These eggs are washed several times in running tap water to remove the traces of sodium hypochlorite. The traces of sodium hypochlorite could be neutralised by dipping the eggs in 10 % sodium thiosulphate solution, and again the eggs are washed thoroughly under running tap water. The surface-sterilised eggs are kept in plastic tubes (7.5 25 cm) on moist tissue paper for continuing the stock culture. After 3 days, the newly hatched larvae are transferred to bouquets of castor leaves and kept in a plastic container with stand for pupation. The pupae are collected 3 days after all the larvae enter the sand. The pupae are sexed and kept on a lid over a wet sponge in adult emergence cage. After 10 days, freshly emerged males and females are collected from their respective emergence cages. Tobacco caterpillar larvae can be multiplied on a chickpea-based semisynthetic diet composition and preparation of which has been mentioned above. Gram pod borer (Helicoverpa armigera): The culture of gram borer is initiated by collecting the adults with the help of light traps. It could be by collection of larvae on a large scale A.S. Kalawate from its host crops in endemic areas. Nucleus culture can also be obtained from the established laboratories. The material thus obtained is reared in the laboratory in aseptic conditions, and the healthy progeny is selected and established. The production starts with the availability of 250 pairs of adults every day, which will yield 10,500 eggs daily. The adults are kept at 100 pairs in each oviposition cage with a cloth enclosing the frame. A circular plastic mesh (on which cotton swabs soaked in water and honey solution are placed in small containers) rests on a support above the base of the frame. The cloth cover is open at both ends with a 20 cm vertical slit in the centre which can be closed with a zip or cloth clips. The cloth cover enclosing the frame is tied with rubber bands at both ends. It is placed on tray with a sponge at the bottom soaked in water. The temperature inside the cage is maintained at 260 C and humidity at 60–90 %. The eggs are laid all over the inner surface of the cloth cover. The egg cloth is removed daily. This cloth is surface sterilised in 10 % formalin for 10 min; the eggs could also be surface sterilised using 0.2 % sodium hypochlorite solution for 5–7 min and treated with 10 % sodium thiosulphate solution to neutralise the effect of sodium hypochlorite and rinsed in distilled water. The eggs are later placed on paper towel under laminar flow for drying. The dried cloth pieces containing eggs are kept in 2 l flasks containing moist cotton. Flasks are plugged with cotton wrapped in muslin cloth, and the bottom of the flask is wrapped with aluminium foil. 4.12.2.3 Rearing of Larvae on Semisynthetic Diet 4.12.2.3.1 Tobacco Caterpillar Stage I (rearing of early instar larvae): The rearing unit is prepared by placing a sponge piece on a glass sheet. The sponge is covered with a single layer of soft tissue paper. A small plastic container containing 200 surface-sterilised eggs of tobacco caterpillar is placed in the centre over the tissue paper. A Petri dish containing about 200 ml of diet is placed inverted over the tissue paper. The eggs hatch within 25 h, and neonate larvae crawl and spread out on the diet. 4 Viral Insecticides Stage II (rearing of late instar larvae): Late instar larvae are reared in modified plastic boxes. One window each on the four sides of the box is cut and covered with a fine plastic mesh to provide sufficient ventilation and to prevent moisture accumulation inside the box. A thick layer of sterilised sand is spread at the bottom of the box. A small piece of tissue paper is kept at the centre over the sand. The diet in the Petri dish (containing 200 larvae) is divided into five equal pieces. One piece of diet bearing 40 larvae is kept in plastic box over the tissue paper so that the sand does not soil the diet. In this way, five boxes are charged with larvae from 1 Petri dish. A plastic grill is fitted into the box in such a manner so that it forms a crest higher than the brim of the box. Thick cake of diet (about 500 g) in a Petri dish is divided into two equal pieces. One such piece is kept on the top of the crest, and the lid of the box is then fixed so that the diet and grill crest are opposed to each other just beneath the lid. After consuming the small quantity of diet on tissue paper, the larvae crawl and perch on the grill and feed from the ceiling of the box. The boxes are stacked and left intact for 3 days. During this time, the diet is almost completely consumed. Now another piece of fresh diet (about 250 g) is kept on the crest in each box, and the boxes are closed and stacked again. During the last 3–4 days of larval stage, the food consumption is higher and so is the faecal matter accumulation on the sand layer. After 20 days from hatching, the larvae move into the sand and start pupating. In a period of 25 days, all the larvae pupate and the chitinisation of pupae is also completed. The boxes are now ready for the pupal harvest. The pupae are collected, cleaned, sterilised and placed in adult emergence cages. The freshly emerged moths are then placed in oviposition cages. Gram borer: The larvae of gram borer can also be reared on a chickpea-based semisynthetic diet as mentioned above. The diet is poured as per the requirement either on the nylon mesh for rearing 5–7-day-old larvae or in tray cells for rearing the older larvae or poured into sterilised Petri plates and allowed to solidify. The diet could be stored in the refrigerators for up to 2 weeks. For preparing large quantities of diet, 63 the quantity of diet ingredients to be used should be calculated accordingly, and industrial-type waring blenders could be used. The larvae are removed from the top of the aluminium foilwrapped flasks with a brush and then transferred to the diet. Two hundred twenty larvae are transferred to diet impregnated on nylon mesh and placed in plastic containers or sterilised glass vials. 100 such containers are maintained daily for 5–7 days. Multicellular trays with semisynthetic diet are advantageous for rearing a large number of larvae. Starting with 10,500 eggs, the total number of larvae available is 10,000 considering an estimated 5 % mortality in initial 5 days of emerging and 10 % mortality up to first 5–7 days. The total number of larvae available for virus production is 8,000 (80 %). The rest of 20 % will be utilised for maintenance of host culture continuously. The diet requirements at various stages of production of larva are as follows: for the young larvae, up to 5–7 days will be 2 g/larva; for 5–7day-old larvae, for Ha NPV production will be 4 g/larva; for 5–7-day-old larvae, for continuation of host culture will be 6 g/larva; and for rearing the field-collected larvae for augmenting the nucleus stock will be about 1 kg. In host culture units, larvae start pupating when they are 18–19 days old, and the pupation will be over within 2–3 days. The harvested pupae are surface sterilised using 0.2 % sodium hypochlorite solution followed by washing with 10 % sodium thiosulphate solution to neutralise sodium hypochlorite and then washed thoroughly with distilled, sterilised water. After washing, the eggs are dried by rolling over blotting paper. The male and female pupae are separated out and placed over moist sponge in adult emergence cages. The egg, larval, pupal and adult stages of gram borer last 3–4, 18–29, 7–8 and 7–9 days, respectively. The oviposition period of the females is about 5 days. 4.12.2.4 Production of Helicoverpa armigera NPV (Ha NPV) and Spodoptera litura NPV (Sl NPV) For Ha NPV and Sl NPV production, the synthetic diet prepared in the laboratory is poured at 4 g/cell in the multi-cavity trays, and the diet surface is uniformly sprayed with virus prepared in distilled 64 A.S. Kalawate Table 4.3 Commercially available products in India Virus Helicoverpa armigera NPV Spodoptera litura NPV Products (company name) Helicide (Pest Control India Ltd., India) Virin-H Helocide Biovirus-H (Biotech International Ltd., India) Helicop Heligard (Margo Biocontrols Pvt. Ltd., India) Spodo-Cide (Pest Control India Ltd., India) Spodoterin Spodi-Cide Biovirus-S (Biotech International Ltd., India) Targets Helicoverpa armigera Spodoptera litura Note: Some of the above-mentioned products are locally made, and hence the formulator name is not known and has not been registered Source: CIB and RC website, minutes of the Registration Committee meetings, June 2003 – March 2009. Other products should be included sterilised water at 18 106 POBs/ml. Eighty percent of the total 5–7-day-old larvae can be utilised for Ha NPV and Sl NPV production. The trays are incubated at 26 C for 7 days. In case of virusinfected larval trays, the diseased larvae die after attaining their maximum size of 6th instar, where the dead caterpillar will have 2–6 billion polyhedral occlusion bodies (POBs), in terms of larval equivalent (LE). One LE of H. armigera NPV ¼ 6 109 POBs; 1 LE of S. litura ¼ 2 109 POBs. The dead larvae have to be harvested, macerated in distilled/sterilised water and filtered through muslin cloth to get the crude suspension of the virus. The extraction is centrifuged to further clarify the solution (Table 4.3). 4.12.3 Other Important Aspects General precautions to be followed while maintaining host cultures are the following: (a) In production units, keep the host culture in a separate room, and the virus production and storage facility should be located in a different facility. (b) In the NPV production units, in spite of best care, 100 % larvae are not infected; the larvae which do not turn inactive after 4–5 days and keep consuming the normal diet should be culled out regularly from the NPV production unit. (c) Utmost care should be taken to prevent the break in the chain of the production system. This could be achieved only if highly dedicated and disciplined workers are engaged for such production units. (d) Strict hygiene should be maintained in different facilities. The equipments used should be either heat sterilised or sterilised using steam or chemicals. The workplace should be thoroughly disinfected with sodium hypochlorite solution. (e) The host culture should be initiated from a batch of healthy adults. (f) Microbial infection could be avoided if good insect husbandry practices are followed. If infection is detected, the culture or infected part should be destroyed immediately. Besides hygienic conditions, optimum temperature (24–26 C) and humidity (65–70 %) should also be maintained. (g) The texture and quality of the natural/semisynthetic diet should be good. (h) entry to host culture unit after visiting virus production unit should be avoided. 4.12.4 Mechanism of Action The virus acts as a stomach poison. The NPV particles are called as polyhedral inclusion bodies (PIBs). When these PIBs present on the plant foliage, the insect larvae will eat the contaminated food, and the virus enters the midgut of the insect larvae. Then the proteinaceous polyhedra come in contact with the alkaline pH of the midgut. The proteinaceous covering rapidly dissolves, thereby releasing the infectious virions. After the 4 Viral Insecticides liberation of virus particles, the nucleocapsid envelop fuses with microvillar membrane of the gut wall cells. The nucleocapsids are released to enter the nucleus where viral DNA replicates and produce secondary infections which invade fat body and the haemolymph. The massive destruction of body tissue eventually kills the insect. 4.12.5 Field Application and Dosage Ha NPV is used for controlling H. armigera attacking cotton, red gram, Bengal gram, tomato, okra, sunflower, groundnut, chillies, maize, sorghum, etc., whereas Sl NPV is used for controlling tobacco caterpillar attacking tobacco, groundnut, soybean, sunflower, cotton, cabbage, beetroot, cauliflower, etc. 4.12.5.1 Directions for Use of NPV The recommended dosage is 200 ml of NPV/acre or 500 ml/ha containing 100 and 250 larval equivalent (LE) of NPV, respectively, as active infective material (one LE ¼ 6 109 POBs), or 100 ml of NPV could be diluted in 200–400 l of water when high volume sprayer is used and in 50–70 l of water in case of power sprayers or preferable to spray using high volume knapsack sprayer. Virus should be sprayed during evening hours. Spray should be initiated as soon as some newly hatched larvae are observed or 3–5 days after a trap catch of 5 months per pheromone trap. Subsequent sprays should be made at 7–10 days intervals depending upon the pest population. 4.12.5.2 Compatibility with Other Insecticides The viral pathogens seem to be less sensitive to chemical pesticides. When the combination of pathogen and pesticide is used, sometimes synergistic action is noticed. But in recent years, mixing of NPV with insecticides is not advisable due to cross resistance problem. 4.12.5.3 Environmental Factors Affecting the Action of NPV The environmental factors which affect the action of NPV are ultraviolet component of 65 sunlight, rainfall, temperature, humidity and leaf surface compounds. The application of Ha NPV in the evening hours provides better efficacy than the morning hours in the field. NPV degrades in the sunlight quickly, and hence adjuvants have to be added along with it while spraying. Solar radiation affects the field persistence of baculovirus. Ultraviolet radiation in the range of 280–310 nm inactivates baculovirus. Fluorescent brighteners can be used to increase its persistence in the field. Leucaena leaf extract, eucalyptus leaf extract and Ranipal can be used with Ha NPV to enhance its efficacy (Kalawate et al. 2005). Temperature in the range of 70–80 C inactivates the viruses for the exposure of 10 min. But the temperature in the field will not reach to this extent, and hence it is less important in the field persistence. Washing of virus from the leaves by rain is a major factor affecting the persistence, and hence stickers have to be added with the viruses. Organic and inorganic substances can be leached from the foliage surface which can have positive or negative effect on the viruses. 4.12.6 Advantages in Using Viral Pesticide Advantages are (1) control of target pests; (2) a high degree of specificity, which makes them especially valuable for use in integrated pest management programmes; (3) safe to humans and other warm-blooded animals; (4) residues present after application of viral pesticides pose no hazards to humans or other animals; and microbial insecticides can be applied even when a crop is almost ready for harvest. 4.12.7 Disadvantages in Using Viral Pesticide Disadvantages include the following: (1) Toxic to only a specific species or group of insects, each application may control only a portion of the pests present in a field, garden or lawn. If other types of pests are present in the treated area, they 66 A.S. Kalawate will survive and may continue to cause damage. Conventional insecticides are subject to similar limitations because they too are not equally effective against all pests. Nonetheless, the negative aspect of selectivity is often more noticeable for microbials. (2) Inactivation by heat, UV and rain can wash out the virus present on the foliage. (3) special formulation and storage procedures are necessary. 4.13 Future Focus In India, the potential of baculovirus has not been utilised fully to control the economic insects. The new developments in this field depend upon the development of recombinant baculoviruses and its commercial production. The most important aspect is to educate the farmers about the benefits of the NPVs. The inclusion of baculoviruses in organic farming and integrated pest management has to be made understood by the farmers. 4.14 Conclusions Baculoviruses provide a promising alternative approach to pest control. Available data suggest that the viruses are effective against insects and do not pose any deleterious effects on other components of the ecosystem (other invertebrates, plants and vertebrates including man). In India, some preliminary work has been done in molecular characterisation of certain indigenous baculoviruses and expression of mostly foreign gene products of medical and veterinary importance utilising baculoviruses of certain alien origin; no work has been done in the field of agricultural plant protection, especially towards genetic improvement of the baculoviruses. References Biji CP, Suheendrakumar W, Sajeev TV (2006) Quantitative estimation of production of Hyblaea puera NPV in three stages of teak defoliator. J Virol Methods 136:78–82 Blissard GW (1996) Baculovirus–insect cell interactions. Cytotechnology 20:73–93 Boguslaw Szewczyk, Marlinda Lobo de Souza, Maria Elita Batista de Castro, Mauricio Lara Moscardi, Flavio Moscardi (2011) In: Stoytcheva M (ed) Baculovirus biopesticides, pesticides-formulations, effects, fate. ISBN: 978-953-307-532-7, InTech, available from: http://www.intechopen.com/articles/ show/title/baculovirus-biopesticides Bonning BC, Hammock BD (1996) Development of recombinant baculoviruses for insect control. Annu Rev Entomol 41:191–210 Boughton AJ, Obrycki JJ, Bonning BC (2003) Effects of a protease-expressing recombinant baculovirus on nontarget insect predators of Heliothis virescens. Biol Control 28:101–110 Burden JP, Hails RS, Windass JD, Suner MM, Cory JS (2000) Infectivity, speed of kill, and productivity of a baculovirus expressing the itch mite toxin txp-1 in second and fourth instar larvae of Trichoplusia ni. J Invertebr Pathol 75:226–236 Chakraborty S, Monsour C, Teakle R, Reid S (1999) Yield, biological activity and field performance of a wild-type Helicoverpa nucleopolyhedrovirus produced in H. zea cell cultures. J Invertebr Pathol 73:199–205 Chang JH, Choi JY, Jin BR, Roh JY, Olszewski JA, Seo SJ (2003) An improved baculovirus insecticide producing occlusion bodies that contain Bacillus thuringiensis insect toxin. J Invertebr Pathol 84:30–37 Couch JA (1974) An enzootic nuclear polyhedrosis virus of pink shrimp: ultrastructure, prevalence, and enhancement. J Invertebr Pathol 24:311–331 Froy O, Zilberberg N, Chejanovsky N, Anglister J, Loret E, Shaanan B (2000) Scorpion neurotoxins: structure/ function relationship and application in agriculture. Pest Manage Sci 56:472–474 Granados RR, Guoxun L, Dersksen ACG, McKenna KA (1994) A new insect cell line from Trichoplusia ni (BTI-Tn-5B1-4) susceptible to Trichoplusia ni single nuclear polyhedrosis virus. J Invertebr Pathol 64:260–266 Hall DW, Hazard EI (1973) A nuclear polyhedrosis virus of a caddisfly, Neophylax sp.. J Invertebr Pathol 21:323–324 Hammock BD, Bonning BC, Possee RD, Hanzlik TN, Maeda S (1990) Expression and effects of the juvenile hormone esterase in a baculovirus vector. Nature 344:458–461 Harrison RL, Summers MD (1995) Mutations in the Autographa californica multinucleocapsid nuclear polyhedrosis virus 25 KDa protein gene result in reduced virion occlusion, altered intranuclear envelopment and enhanced virus production. J Gen Virol 76:1451–1459 Heimpel AM, Thomas ED, Adams JR, Smith LJ (1973) The presence of nuclear polyhedrosis virus of Trichoplusia ni on cabbage from the market shelf. Environ Entomol 2:72–76 4 Viral Insecticides Henry JE, Jutila JW (1966) The isolation of a polyhedrosis virus from a grasshopper. J Invertebr Pathol 8:417–418 Hoover K, Kishida KT, DiGiorgio LA, Workman J, Alaniz SA, Hammock BD, Duffey SS (1998) Inhibition of baculoviral disease by plant-mediated peroxidase activity and free radical generation. J Chem Ecol 24:1949–2001 Hughes PR, Wood HA, Breen JP, Simpson SF, Duggan AJ, Dybas JA (1997) Enhanced bioactivity of recombinant baculoviruses expressing insect-specific spider toxins in lepidopteran crop pests. J Invertebr Pathol 69:112–118 Ignoffo CM, Couch TL (1981) The nucleopolyhedrosis virus of Heliothis species as a microbial pesticide. In: Burges HD (ed) Microbial control of pests and plant diseases. Academic, London, pp 329–362 Inceoglu AB, Kamita SG, Hinton AC, Huang Q, Severson TF, Kang KD, Hammock BD (2001) Recombinant baculoviruses for insect control. Pest Manage Sci 57:981–987 Inceoglu AB, Kamita SG, Hammock BD (2007) Genetically modified baculoviruses: a historical overview and future outlook. Adv Virus Res 68:323–360 Jacques RP, Morris ON (1981) Compatibility of pathogens with other methods of pest control and crop protection. In: Burges HD (ed) Microbial control of pests and plant diseases. Academic, London Jem KJ, Gong T, Mullen J, Georgis R (1997) Development of an industrial insect cell culture process for large scale production of baculovirus biopesticides. In: Maramorosch K, Mitsuhashi J (eds) Invertebrate cell culture: novel directions and biotechnology applications. Science Publishers, Enfield, pp 173–180 Kalawate A, Nachane MN (2006) Leucaena leaf extract as adjuvant with HaNPV. J Maharashtra Agric Univ 31 (3):367–368 Kalawate A, Nachane MN, Dhondge AS (2005) Efficacy and retentivity of HaNPV under field conditions on cotton. Plant Prot Bull 57(1/2):16–19 Kalmakoff, Ward (2007) Baculoviruses. http://www. microbiologybytes.com/virology/kalmakoff/baculo/ baculo.htm. Accessed 21 Oct 2011 Kamita GK, Maeda S, Hammock BD (2003) Highfrequency homologous recombination between baculoviruses involves DNA replication. J Virol 77:13053–13061 Krell PJ (1996) Passage effect of virus infection in insect cells. Cytotechnology 20:125–137 Kumari V, Singh NP (2009) Spodoptera litura nuclear polyhedrosis virus (NPV-S) as a component in Integrated Pest Management (IPM) of Spodoptera litura (Fab.) on cabbage. J Biopest 2:84–86 Larsson R (1984) Insect pathological investigations on Swedish Thysaura: a nuclear polyhedrosis virus of the bristletail Dilta hibernica. J Invertebr Pathol 44:172 Lua LHL, Pedrini MRS, Reid S, Robertson A, Tribe DE (2002) Phenotypic and genotypic analysis of 67 Helicoverpa armigera nucleopolyhedrovirus serially passed in cell culture. J Gen Virol 83:945–955 McCutchen BF, Hoover K, Preisler HK, Betana MD, Herrmann R, Robertson JL, Hammock BD (1997) Interaction of recombinant and wild-type baculoviruses with classical insecticides and pyrethroid-resistant tobacco budworm (Lepidoptera: Noctuidae). J Econ Entomol 90:1170–1180 Mettenmeyer A (2002) Viral insecticides hold promise for bio-control. Farming Ahead 124:50–51 Miller LK (1997) Introduction to the baculoviruses. In: Miller LK (ed) The baculoviruses. Plenum Press, New York Morales L, Moscardi F, Sosa-Gomez DR, Paro FE, Soldorio IL (2001) Fluorescent brighteners improve Anticarsia gemmatalis (Lepidoptera: Noctuidae) nucleopolyhedrovirus (AgMNPV) activity on AgMNPV susceptible and resistant strains of the insect. Biol Control 20:247–253 Moscardi F (1999) Assessment of the application of baculoviruses for control of Lepidoptera. Annu Rev Entomol 44:257–289 Moscardi F (2007) A Nucleopolyhedrovirus for control of the velvetbean caterpillar in Brazilian Soybeans. In: Vincent C, Goethel MS, Lazarovits G (eds) Biological control: a global perspective. CAB International, Oxfordshire/Cambridge, pp 344–352 Nakamura T (2003) Control of leafrollers in tea fields using Hamakitenteki. http://www.agrofrontier.com/ guide/f_105.html. Accessed 12 Jan 2012 Nishi Y, Nonaka T (1996) Biological control of the tea tortrix using granulosis virus in the tea field. Agrochem Jpn 69:7–10 O’Reilly DR, Miller LK (1991) Improvement of a baculovirus pesticide by deletion of the EGT gene. Biotechnology 9:1086–1089 Pedrini MRS, Nielsen LK, Reid S, Chan LCL (2005) Properties of a unique mutant of Helicoverpa armigera single-nucleocapsid nucleopolyhedrovirus that exhibits a partial many polyhedra and few polyhedra phenotype on extended serial passaging in suspension cell cultures. In Vitro Cell Dev Biol 41:289–297 Pedrini MRS, Christian P, Nielsen LK, Reid S, Chan LCL (2006) Importance of virus-medium interactions on the biological activity of wild-type Heliothine nucleopolyhedroviruses propagated via suspension insect cell cultures. J Virol Methods 136:267–272 Pijlman GP, van den Born E, Martens DE, Vlak JM (2001) Autographa californica baculoviruses with large genomic deletions are rapidly generated in infected cells. Virology 283:132–138 Reardon R, Podgwaite JP, Zerillo RT (1996) GYPCHEK – The gypsy moth nucleopolyhedrosis virus product. USDA Forest Service Publication FHTET-96–16 Roman Georgis (1996) Present and future prospects of biological insecticides. Cornell community conference on biological control-April 11–13, 1996. 68 http://web.entomology.cornell.edu/shelton/cornell-bio control-conf/talks/georgis.html. Accessed 11 Jan 2012 Ryel RB, Cline GB (1970) Isolation and characterization of a nuclear polyhedral inclusion body from the boll weevil, Anthonomus grandis. J Ala Acad Sci 41:193 Shapiro M, Daugherty EM (1994) Enhancement in activity of homologous and heterologous viruses against gypsy moth (Lepidoptera, Lymantriidae) by an optical brightener. J Econ Entomol 87:361–365 Singhal V (2004) Biopesticides for sustainable agriculture: prospects and constraints. In: Kaushik E (ed) Biopesticides in India. All India Biotech Association, VIPPS Centre. New Delhi, pp 31–40 Slavicek JM, Hayes-Plazolles N, Kelly ME (2001) Identification of a Lymantria dispar nucleopolyhedrovirus isolate that does not accumulate few-polyhedra mutants during extended serial passage in cell culture. Biol Control 22:159–168 Souza ML, Castro MEB, Barros AML, Sihler W, Moscardi F (2001) Análise de DNA de isolados de nucleopolyhedrovirus de Anticarsia gemmatalis utilizados no controle da lagarta da soja no Brasil. Boletim de Pesquisa e Desenvolvimento. Embrapa Recursos Genéticos e Biotecnologia Srinivasa M, Jagadeesh Babu CS, Anitha CN, Girish G (2008) Laboratory evaluation of available commercial formulations of HaNPV against Helicoverpa armigera (Hub.). J Biopest 1:138–139 Sun XL, Peng H (2007) Recent advances in biological pest insects by using viruses. Virol Sin 22:158–162 Sun X, Wang H, Sun X, Chen X, Peng C, Pan D (2004) Biological activity and field efficacy of a genetically modified Helicoverpa armigera SNPV expressing an A.S. Kalawate insect-selective toxin from a chimeric promoter. Biol Control 29:124–137 Szolajska E, Poznanski J, Ferber ML, Michalik J, Gout E, Fender P, Bailly I, Dublet B, Chroboczek J (2004) Poneratoxin, a neurotoxin from ant venom. Structure and expression in insect cells and construction of a bio-insecticide. Eur J Biochem 271:2127–2136 Van Oers MM (2006) Vaccines for viral and parasitic diseases produced with baculovirus vectors. Adv Virol Res 68:193–253 Vincent C, Andermatt M, Valéro J (2007) Madex and VirosoftCP4®, viral biopesticides for coddling moth control. In: Vincent C, Goethel MS, Lazarovits G (eds) Biological control: a global perspective. CAB International, Oxfordshire/Cambridge, pp 336–343 Wahab S (2004) The Department of Biotechnology initiates towards the development and use of biopesticides in India. In: Kaushik E (ed) Biopesticides for sustainable agriculture: prospects and constraints. The Energy and Resources Institute, New Delhi, pp 73–90 Zhang GY, Sun XL, Zhang ZX, Zhang ZF, Wan FF (1995) Production and effectiveness of the new formulation of Helicoverpa virus pesticide-emulsifiable suspension. Virol Sin 10:242–247 Zhou MZ, Sun HC, Hu ZH, Sun XL (2004) SOD enhances infectivity of Helicoverpa armigera single nucleocapsid nucleopolyhedrosis against H. armigera larvae. Virol Sin 18:506–507 Zou Y, Young SY (1994) Enhancement of nuclear polyhedrosis virus activity in larval pests of Lepidoptera by a stilbene fluorescent brightener. J Entomol Sci 29:130–133