9SciGlowBurningSplitLabLessonPlan
advertisement

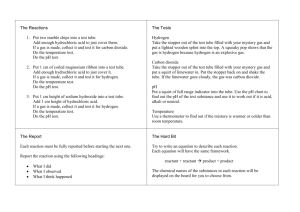
Lesson Plan Template SUBJECT/Grade: Grade 9 Academic Science COURSE/Type/Code: SNC 1D LESSON TITLE : Suggested Time: 75 mins Lab – Identifying Mystery Gases (burning/glowing stint) LESSON Description: Air is a mixture of gases, including nitrogen, oxygen and carbon dioxide. All of the gases contain covalent bonds. Most of the gases in air are colourless and odourless. Although these and other gases are indistinguishable in appearance, they exhibit different chemical properties. These properties can be used to identify them. Connection to CULMINATING ACTIVITY: Students will be given a handout to fill in observations and discussion questions – this is testable material Planning Information: Curriculum Connections Overall Expectation(s): C2.2 Conduct an inquiry to identify the physical and chemical properties of common elements and compounds (e.g., magnesium, HCl, NaHCO3, Manganese dioxide) C2.4 Conduct appropriate chemical tests to identify some common gases (e.g., oxygen, hydrogen, carbon dioxide) on the basis of their chemical properties, and record their observations Specific Expectation(s): Covalent bond, triple bond, chemical bond, double bond, molecule, ion, electronegativity, ionic bond, anion, cation, nonpolar covalent bond, hydrogen bond, polar covalent bond, and polar Octet Rule, ion formation, ion names, ion formulas, ionic compound formation, ionic compound properties, lattice energy, simple binary compounds, compounds with polyatomic ions, compounds with transition metals, covalent compound formation, covalent compound properties, and binary covalent compounds Learning Goal(s) or Enduring Understandings: Explain the stability of noble gases by electron configuration Identify elements that can release or receiving valence electrons to achieve stability or resembling the noble gas electron configuration. Describe the arrangement of valence electrons noble gas atom (duplet and octet) with the arrangement of valence electrons instead of the noble gas atom and its relationship with element stability Given examples of elements which readily releases its valence electrons to form a positive ion and example elements that readily accept electrons from the valence ions of other elements to form negativ element. Illustrates the process of ionic bonding, such as men (positive charge) and female(negative charge) are attracted to form anionic bond of marriage (a sense of intending to know) Essential Questions: Can I define chemical bonding and different types of bonds? Do I know how to identify and demonstrate the different types of chemical bonds through Lewis Dot structures Prior Knowledge Required (the knowledge/concepts and skills students must possess to be successful in this lesson) Bohr Rutherford diagrams Lewis dot Subatomic particles and atomic structure Periodic table groups/families Differentiated Instruction Details How will you differentiate your lesson? Provide details Knowledge of Students Differentiation based on student: Readiness Interests Learner Profile: Styles Intelligences environment, Other (e.g., gender, culture) Need to Know Students’ come from different cultural backgrounds and language use at home – the terms need to be defined clearly and broken down Many of the students have not been doing homework – and so readiness may not be there (Flexible grouping is a strategy that teachers can use to create temporary groups based on student need) How to Find Out Multiple intelligence tests? Differentiated Instruction Response Learning materials (content) Ways of learning (process) Ways of demonstrating learning (product) Learning environment Resources (for items in appendix, indicate with asterisk) Projector/laptop Chart paper/markers Agenda (to be listed on blackboard, in student language) Homework: Handout from Friday (finish) and Textbook page 257-261 (and questions at the end) Minds On (Hook) Establishing a positive learning environment Connecting to prior learning and/or experiences Connections L: Literacy AfL, AoL: Assessment for/of Learning Setting the context for learning Volunteers (4) CAN WE DO DIAGRAMS? (20 minutes) Have four volunteers for board work - Two do the Bohr- Rutherford diagram for Na and Cl - Two do the Lewis Dot diagram for Na and Cl Show the students the reaction - Show video of NaCl bonding - https://study.com/academy/lesson/what-is-sodiumchloride-definition-structure-formula.html Action AoF Work through example with students to ensure everyone understands (terms and how to form diagrams) Introducing new learning or extending/reinforcing prior learning Providing opportunities for practice and application of learning (guided > independent) AfL Whole Class PowerPoint presentation 30 mins Go through examples on the board of chemical bonding - PowerPoint – present material to whole group If there is time: - Present ions and ask how they would bond – if ionic (which would be the anion or cation), if covalent (why?) - Examples: Show the formation of ionic bonding in MgO compound! Consolidation and Connection Helping students demonstrate what they have learned Providing opportunities for consolidation and reflection Use images and not just works on powerpoint Bold and underlnes things that are key terms or important things to write down Groups of 5 Let's Laugh About It (20 mins) Help students remember the four different types of chemical bonds by creating jokes about their characteristics. 1. Describe the four types of chemical bonds: covalent, ionic, polar covalent, and metallic. Draw diagrams to illustrate common compounds that have each type of bond. 2. As a class, create a four-way Venn diagram to compare and contrast the characteristics of the four bonds. 3. Divide the class into pairs, and provide each pair with chart paper and markers. 4. Have pairs create a joke to help them remember something about each type of chemical bond. For example, a joke might be: ''Why are covalent bonds good citizens? Because they always share their electrons.'' 5. Students should write their jokes on the chart paper. 6. When students are finished, have them share their jokes with the class. Extension/PREP/Hwk (activities completed outside of class to reinforce/extend learning or prepare for next class) AoL: See if the students understand the terms of bonding enough to make jokes in the periodic table AfL or AoL: Strategy/Assessment Tool Page 261 in textbook Accommodations/Special Needs: (this may have been identified above in DI section) How will you accommodate for students with IEPs, ELLs etc.? Allowed to use their phones even IN PAIRS to make chemistry memes Can use google translate Teacher Reflection on Lesson: (to be completed after teaching, you do not need to fill this out for this assignment, just an FYI for reflective practice) Aspects that worked: It appears the first period class does well with noise as well as silence – they are able to sit down and work given clear direction but prefer lecture based learning or lab work Changes for next time: https://study.com/academy/lesson/chemical -bonding-activities-games.html Have students present examples of Bohr Rutherford Diagrams Examples in groups: Group 1: LiF, H2O, MgCl2, HCl, CH4, O2 SNC1D MRS. WHALEN LAB: IDENTIFYING MYSTERY GASES INTRODUCTION: Air is a mixture of gases, including nitrogen, oxygen and carbon dioxide. All of the gases contain covalent bonds. Most of the gases in air are colourless and odourless. Although these and other gases are indistinguishable in appearance, they exhibit different chemical properties. These properties can be used to identify them. Oxygen: Oxygen is essential for burning. Things burn more vigorously when they are in pure oxygen than when they are in air, which contains about 20% oxygen. Hydrogen: When hydrogen is ignited in air, the hydrogen atoms combine with oxygen atoms to form molecules of water vapour. This rapid reaction is accompanied by a characteristic “pop” sound. Carbon Dioxide: Carbon dioxide does not burn and does not support combustion. Carbon dioxide with extinguish a flame, making it useful as a fire extinguisher. PURPOSE: The purpose of this lab is to use the information on gas tests to identify different gases produced in the reactions. MATERIALS: Goggles 3 test tubes test-tube rack hydrogen peroxide solution manganese dioxide powder toothpick 3 wooden splints Bunsen burner Flint starter Hydrochloric acid solution Magnesium ribbon Tongs Sodium bicarbonate powder SAFETY PRECAUTIONS: Hydrochloric acid is corrosive. Any spills on the skin, in the eyes, on clothing should be washed immediately with cold water. Report any spills to your teacher. Hydrogen peroxide is poisonous and a strong irritant. Manganese dioxide is also toxic. Report any spills to your teacher. PROCEDURE: 1. 2. 3. 4. 5. 6. Part 1: Hydrogen Peroxide and Manganese Dioxide Put on your goggles. Using a clean, dry test tube, pour about 4 cm of hydrogen peroxide solution into a test tube. Obtain a tiny amount of manganese dioxide powder on the blunt end of a toothpick. Record 3 physical properties of the two reactants in the observation table. Light a burning splint using the Bunsen burner according to the instructions given by your teacher. Add the manganese dioxide to the hydrogen peroxide. Allow the reaction to proceed for about 5 seconds, and note changes during the reaction in the observation table. Bring the burning splint close to the mouth of the test tube. If no reaction occurs, blow out the flame and insert the glowing splint half way into the test tube. Record your observations, and the result of the splint test in the observation table. Part 2: Hydrochloric Acid and Magnesium 1. Obtain another clean, dry test tube. Pour about 4 cm of hydrochloric acid solution into the test tube. Obtain a small piece of magnesium ribbon. Record 3 physical properties of the two reactants in the observation table. 2. Light a burning splint using the Bunsen burner according to the instructions given by your teacher. 3. Roll the magnesium into a ball and add it to the acid using forceps. Note any changes of the reaction in the observation table. 4. Bring the burning splint close to the mouth of the test tube. If no reaction occurs, blow out the flame and insert the glowing splint half way into the test tube. 5. Record your observations, and the result of the splint test in the observation table. 1. 2. 3. 4. 5. Part 3: Hydrochloric Acid and Sodium Bicarbonate Obtain one clean, dry test tube. Pour about 4 cm of hydrochloric acid solution into the test tube. On a piece of paper, obtain a small amount of sodium bicarbonate. Record 3 physical properties of the two reactants in the observation table. Slowly add the sodium bicarbonate into the test tube containing hydrochloric acid. Note any changes of the reaction in the observation table. Light a burning splint using the Bunsen burner according to the instructions given by your teacher. Bring the burning splint close to the mouth of the test tube. If no reaction occurs, blow out the flame and insert the glowing splint half way into the test tube. Record your observations, and the result of the splint test in the observation table. Dispose of all mixtures into the waste containers provided. Clean up your workstation, put away all materials and wash your hands. DISCUSSION QUESTIONS: Answer the following questions using complete sentences. 1. What gas(es) were you testing for with the burning splint? The glowing splint? (2 marks) 2. What evidence of chemical change did you observe with each reaction? (3 marks) 3. Which gas seemed to be the most hazardous in this activity? Why? (2 marks) 4. Fill in the following table, listing the chemical and physical properties of the gases produced in this investigation. (6 marks) Gas Hydrogen Carbon Dioxide Oxygen Physical Properties (2) Chemical Properties (2) PART NAME STARTING SUBSTANCE PROPERTIES (AT LEAST 3) OBSERVATIONS AFTER MIXING (AT LEAST 3) RESULTS OF GAS TEST 1 Manganese Dioxide Burning: Glowing: Hydrogen Peroxide 2 Magnesium Burning: Glowing: Hydrochloric Acid 3 Sodium Bicarbonate Burning: Glowing: Hydrochloric Acid GAS PRODUCED