2017 chemistry external exam report
advertisement

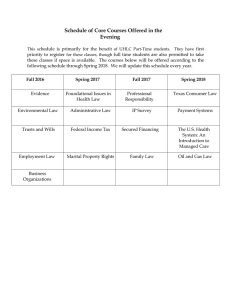
Chemistry 2017 Senior External Examination: Assessment report Statistics Year Number of candidates Level of achievement VHA HA SA LA VLA 2017 20 3 6 6 3 2 2016 12 0 0 4 4 4 2015 22 2 0 4 7 9 2014 21 3 1 2 7 8 2013 27 4 2 4 11 6 General comments Paper One Part A assessed Knowledge and simple application and consisted of 10 multiplechoice questions and eight short-response items covering all eight syllabus topics. Marks were allocated in proportion to syllabus topic weightings. Paper One Part B assessed Scientific processes and contained five questions assessed against specific criteria. Candidates were required to respond to any four of the five questions. Paper Two assessed Complex reasoning processes and contained five questions assessed against specific criteria. Candidates were required to respond to any four of the five questions. Paper One Part A — Knowledge and simple application Short-response questions This part of the examination required candidates to demonstrate their knowledge and ability in simple application of the eight syllabus topics. Question 11 a. The majority of candidates successfully identified the chemical term associated with the supplied definitions. b. Most candidates could distinguish between the two materials. 180120 c. Most candidates could write some correct chemical formulas. d. Most candidates could identify the structure of a covalent bond. e. The numbers of neutrons and electrons were easily identified. f. Shapes and polarities of molecules were not well understood. g. Bonding within and between molecules is still challenging for many candidates. Question 12 a. It was evident that many candidates had used the terms interchangeably and failed to appreciate any difference. b. Most candidates were familiar with molarity. c. Many candidates successfully calculated the mass present. d. Conversion of mass to moles to volume was challenging for many candidates. e. Most candidates experienced difficulty with empirical formula determination. Question 13 a. Most candidates determined the required oxidation number. b. Most candidates successfully calculated the cell potential. c. Many candidates used Faraday’s laws to calculate the mass deposited. d. The diagram of the electrochemical cell often contained incorrect locations of anode and cathode and consequently incorrect electron flow. Question 14 a. Most candidates could select the appropriate chemical terms. b. Very few candidates could correctly name the given alcohol. c. Many candidates could make substantial progress in determining the structural formulas. Question 15 a. Most candidates correctly stated the electron configurations. b. Some candidates could link ionisation energies with element location in the periodic table. c. Almost all candidates could link electron configuration to periodic table group. d. Many candidates understood the factors determining differences in chemical bonding. Question 16 a. Many candidates could not list the importance of nitrogen gas. b. Many candidates could list the assumptions of kinetic theory. c. Real gases were not understood by most. d. Most candidates could recall the general gas equation but were then unable to calculate the moles of the gas. Question 17 a. Most candidates could identify the change in energy. b. Most candidates had some knowledge of reaction rates. c. Most candidates could not identify the need for the activation energy to be achieved. Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 2 of 34 d. Many candidates were able to successfully calculate the energies associated with bond breaking and bond formation but then confused the direction of energy flow. e. Many candidates made substantial progress once the required equation was identified. Question 18 a. Many candidates understood the concept of ‘weak acid’. b. Some candidates could appreciate that forward and reverse reactions were proceeding at equal rates even though macroscopic properties were constant. c. Many candidates could write the required equations. d. Most candidates did not understand the meaning of an equilibrium constant. e. Most candidates could link stresses to the subsequent changes to equilibrium concentrations. f. Most candidates could not apply knowledge of acids and bases to determine the required pH. Candidates are encouraged to make use of prior examination papers (and their worked solutions) in order to gain a greater appreciation of the range and depth of tasks appropriate to the various syllabus topics. Part B — Scientific processes Candidates were directed to respond to four of the five questions. All candidates complied with this directive. It was evident that most candidates devoted enough time to this section. Question 1 The novel presentation of the fundamental concept of half-cell reduction potentials was not attempted by many candidates. Question 2 Many candidates were able to make substantial progress in the analysis of data based on a Charles Law experiment. Question 3 Many candidates were able to successfully analyse and evaluate the information relating to materials, their bonding and their properties. Question 4 Many candidates attempted this question and could successfully plot the given data. The concepts of limiting and excess reagents were understood by most. Question 5 Candidates were familiar with factors affecting reaction rates and many could provide explanations that demonstrated understanding. Scientific processes is one of the three Chemistry criteria and it is important that candidates demonstrate an ability to successfully engage in simple scientific process tasks by processing Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 3 of 34 information, making simple judgments and communicating information in various forms throughout their course of study. Paper Two — Complex reasoning processes In responses to Complex reasoning processes questions, candidates must demonstrate logical reasoning and critical thinking with reference to the chemistry applicable to the task. Credit cannot be given for recall of knowledge or simple application of that knowledge. Question 1 Candidates were often able to reduce the given information to a series of steps, each involving a calculation of moles of either product or reactant. Question 2 Candidates often were unable to use half reactions to generate the balanced equation for the redox process. Question 3 Only two candidates made substantial progress in matching the trial Ksp with the actual Ksp. Question 4 Candidates were often able to calculate the moles of carbon and hydrogen, but then experienced difficulty in the calculation of the molar mass and subsequent molecular formula. Naming of the unfamiliar carboxylic acids was uncertain. Question 5 Candidates were provided with an equilibrium dissociation position rather than a standard equilibrium constant. This challenged candidates in their calculations of moles present at equilibrium and subsequent volume of gas mixture. Candidates are encouraged to develop an understanding of chemical principles and processes, rather than attempting to commit huge amounts of factual information to memory. Only then will they be able to successfully use complex reasoning in challenging and often unfamiliar situations. Marker responses The sample solutions on the following pages show possible ways of successfully responding to the questions. It must be stressed that they do not provide the only valid responses. Other approaches and problem-solving strategies may be equally valid. In Complex reasoning processes, the valid response from a candidate must include the demonstration of logical reasoning and critical thinking, with reference to the chemistry applicable to the task. No credit was given to recall of knowledge or simple application of that knowledge. Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 4 of 34 Paper One Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 5 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 6 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 7 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 8 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 9 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 10 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 11 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 12 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 13 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 14 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 15 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 16 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 17 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 18 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 19 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 20 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 21 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 22 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 23 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 24 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 25 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 26 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 27 of 34 Paper Two Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 28 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 29 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 30 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 31 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 32 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 33 of 34 Chemistry 2017 Senior External Examination: Assessment report Queensland Curriculum & Assessment Authority March 2018 Page 34 of 34