Solid State Physics: Crystal Structure Lecture Notes
advertisement

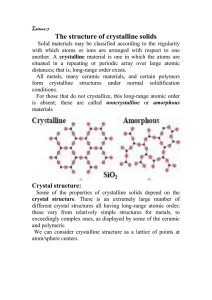
Solid State Physics - I Dr. Ram Chand February 10, 2019 ii Contents 1 Crystal Structure 1.1 Introduction . . . . . . . . 1.1.1 Solid State Physics 1.1.2 Classification . . . 1.1.3 Crystallography . . 1.1.4 Crystal Structure . 1.1.5 Terminology . . . . . . . . . . . . . . . . 2 The Second Chapter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 1 1 1 2 2 2 5 iii iv CONTENTS Chapter 1 Crystal Structure 1.1 1.1.1 Introduction Solid State Physics Solid-state physics is the study of rigid matter, or solids, through methods such as quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. 1.1.2 Classification The solids may be broadly classified as crystalline and non-crystalline (amorphous) depending upon the arrangement of atoms or molecules. CRYSTALLINE SOLIDS The crystalline state of solids is characterized by regular or periodic arrangement of atoms or molecules. Crystalline solids may be sub-divided into single crystals and polycrystalline solids. Single Crystal In single crystals, the periodicity of atoms extends throughout the material as the case of diamond, quartz, mica, etc. Polycrystalline A polycrystalline material is made up of smaller crystallites. Each small crystallite is known as grain and there grain boundaries between two 1 2 CHAPTER 1. CRYSTAL STRUCTURE grains. Single cystals may be classified as elemental crystal (e.g., Al. Fe, Cu, etc.) and ionic crystal (AgCl, CuSO4 , etc.). AMORPHOUS SOLIDS The non-crystalline or amorphous solids are characterized by the completely random arrangement of atoms or molecules. These solids exhibit short range order. Glass is an example of amorphous materials. Most of the plastics and rubbers are also amorphous. 1.1.3 Crystallography The science which deals with the study of geometrical forms and physical properties of crystalline solids is called crystallography. 1.1.4 Crystal Structure A crystal is a 3-dimensional body. Regular and periodic arrangement of 3dimensional pattern of atoms or molecules in space, is called crystal structure, where each and every atom or molecule have the same environment. 1.1.5 Terminology Point Lattice An arrangement of infinite number of imaginary points in three-dimensional space with each point having identical surroundings is known as point lattice. Space Lattice A space lattice is defined as an array of imaginary points which are so arranged in space that each point has identical surroundings. Lattice Plane Within crystal any plane passing through at least one lattice point is called a lattice plane. 1.1. INTRODUCTION 3 Basis An atom or group of atoms which is placed on each lattice points in regular fashion is known as basis. The basis acts as building unit or structural unit for a complete crystal structure. It can be expressed as: Space lattice + basis = Crystal Structure A lattice is mathematical concept and crystal is physical concept. Unit Cell A unit cell may be defined as the smallest unit of the lattice which, on continuous repetition, generates the complete lattice. Both primitive and non-primitive translation vectors may be used to construct a unit cell. Primitive unit cell is the smallest volume cell. All the lattice points belonging to a primitive cell lie at its corners. Therefore, the effective number of lattice points in a primitive unit cell is one. A non-primitive cell may have the lattice points at the corners as well as at other locations both inside and on the surface of the cell and, therefore, the effective number of lattice points in a non-primitive cell is greater than one. The distance (a) between two atoms or ions of same type is called the length of unit cell. Lattice Parameter The primitive vectors ~a, ~b, ~c and the inter-facial angles α, β, γ are together called as ”lattice parameter” of the crystal. Lattice Vector and Direction To specify certain point or direction in unit cell, we use lattice vector ~a, ~b, ~c as: T~ = n1~a + n2~b + n3~c where n1 , n2 , n3 are three integers. Symmetry Operation A symmetry operation is that which transforms the crystal to itself, i.e., crystal remains invariant under symmetry operation. These operations are translation, rotation, reflection and inversion. 4 CHAPTER 1. CRYSTAL STRUCTURE Chapter 2 The Second Chapter 5 6 CHAPTER 2. THE SECOND CHAPTER