Physical Vs Chemical Change Lab
advertisement

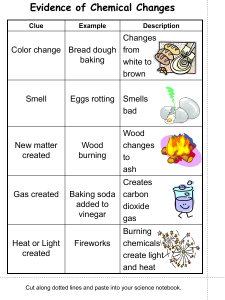
Background When a physical change occurs, only the form of the substance changes. Chemical changes, however, result in the formation of new substances with different properties. Some general signs of a chemical change include a change in color or odor, the formation of a precipitate, formation of a gas, and changes in heat or light. In this lab, you will perform different activities and determine whether a physical or chemical change has occurred based upon your observations. Success Goals Procedures 1. According to the data table below, either mix or perform the actions listed under the “Activity” column. 2. Make a hypothesis (educated guess) about whether you think it will be a chemical or physical reaction. 3. Under the “Observations” column, record any and all observations for each experiment. 4. Under the “Physical/Chemical Change” column, determine whether each experiment resulted in a physical or chemical change. 5. If the experiment resulted in a chemical change, what evidence proves your statement? Data Activity Light a match for ten seconds Burn a candle for two minutes Apple cut in two and left out Mix baking powder with water Mix baking powder with vinegar Hypothesis: Will it be chemical or physical? Observations Physical or chemical? What’s your evidence? How do you know? Mix baking soda with vinegar Egg in vinegar Use tongs to hold an egg over flame “Flame egg” in water Post-Lab Reflection 1) How would you explain the difference between physical and chemical changes to a 1st grader? 2) Describe some ways you can tell that a chemical change has occurred and NOT a physical change. 3) List some physical changes AND chemical changes you find in nature: PHYSICAL CHANGES CHEMICAL CHANGES 1. 1. 2. 2. 3. 3. 4. 4. 4) Which do you think is more common in nature chemical or physical changes and WHY?