Notes - Matter and Change
advertisement
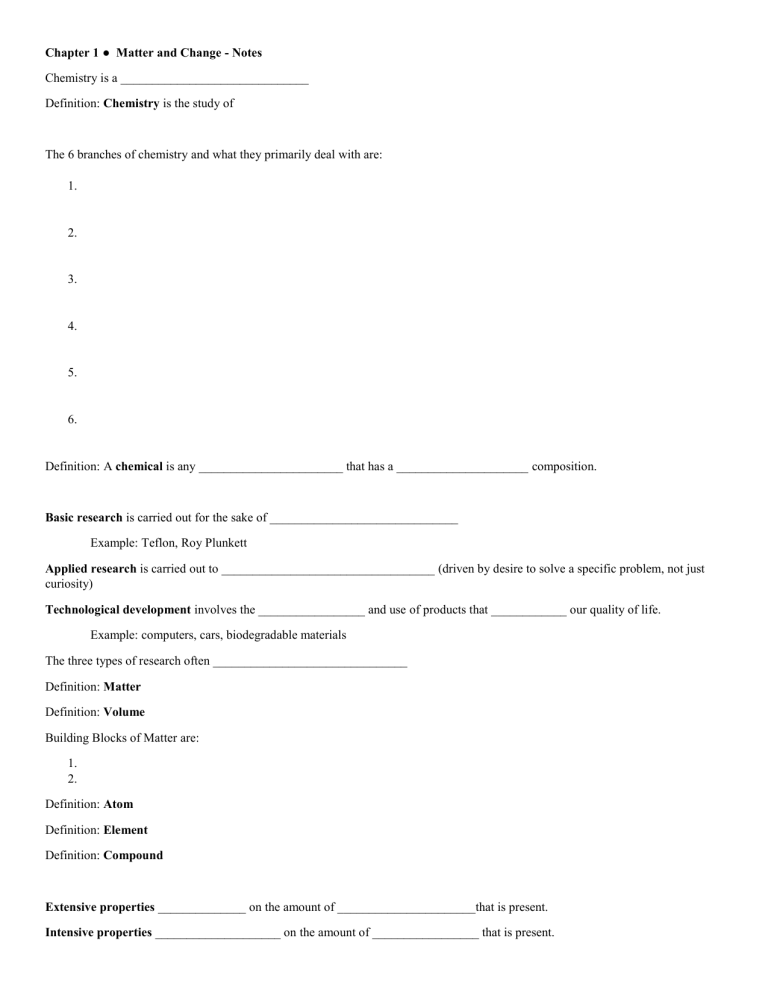
Chapter 1 ● Matter and Change - Notes Chemistry is a ______________________________ Definition: Chemistry is the study of The 6 branches of chemistry and what they primarily deal with are: 1. 2. 3. 4. 5. 6. Definition: A chemical is any _______________________ that has a _____________________ composition. Basic research is carried out for the sake of ______________________________ Example: Teflon, Roy Plunkett Applied research is carried out to __________________________________ (driven by desire to solve a specific problem, not just curiosity) Technological development involves the _________________ and use of products that ____________ our quality of life. Example: computers, cars, biodegradable materials The three types of research often _______________________________ Definition: Matter Definition: Volume Building Blocks of Matter are: 1. 2. Definition: Atom Definition: Element Definition: Compound Extensive properties ______________ on the amount of ______________________that is present. Intensive properties ____________________ on the amount of _________________ that is present. Physical Property –a characteristic that can be __________________ without changing the ____________ of the substance. Ex: Physical change - a change in a substance that does not involve a change in the ______________ of the substance. Ex: A change of state is a ______________of a substance from one state to another. Three common _____________________________ - solid state, liquid state, gas state The solid state has ___________________ and __________________________. The liquid state has a ___________________ but an ______________________shape. The gas state has neither __________________ nor definite ____________. Plasma - is a high-temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. (Found in fluorescent bulbs) Chemical property relates to a substance’s ability to ____________________________________________ _____________________________________________________________________________________ Chemical change - A change in which one or more substances are converted into _____________ substances. Also called a chemical ____________________ Reactants are Products are Energy is ___________________involved when physical or chemical changes occur. Energy can be in various forms. Energy can be ______________ or released in a change, it is ____________ destroyed or created. (Law of Conservation of Energy) Mixtures: Homogeneous mixtures are called _____________________ • _____________________in composition (salt-water solution) Heterogeneous mixtures • ____________uniform throughout (clay-water mixture) Pure Substances: A pure substance has a _____________________composition. Pure substances are either ________________or ____________________. Elements: The vertical columns of the periodic table are called___________ or _____________________. • Each group contains elements with similar chemical properties. The _____________________rows of elements in the periodic table are called periods. Physical and chemical properties change somewhat regularly across a period. A metal is an element that is a good electrical conductor and a good heat conductor. Other Properties: A nonmetal is an element that is a poor conductor of heat and electricity. Other Properties: A metalloid is an element that has some characteristics of metals and some characteristics of nonmetals. Other Properties: Noble Gases – Where are they located on the periodic table??? Other Properties: