Thermodynamics Practice Problem: First Law Analysis
advertisement
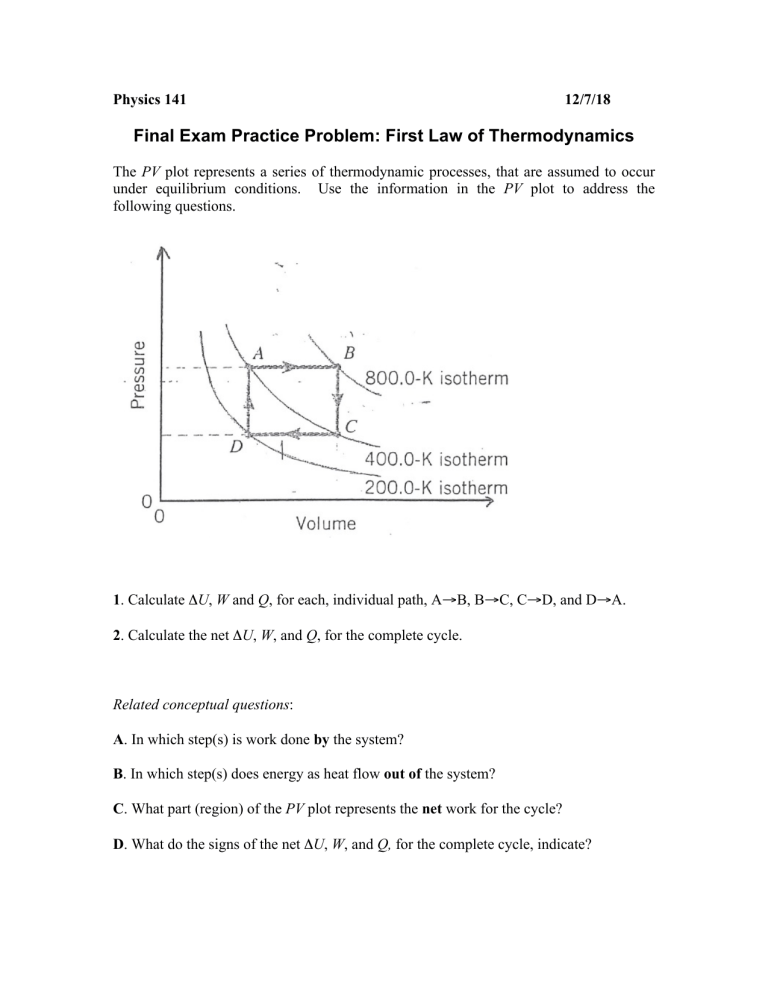
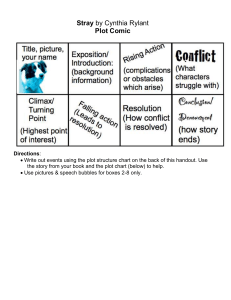
Physics 141 12/7/18 Final Exam Practice Problem: First Law of Thermodynamics The PV plot represents a series of thermodynamic processes, that are assumed to occur under equilibrium conditions. Use the information in the PV plot to address the following questions. 1. Calculate ΔU, W and Q, for each, individual path, A→B, B→C, C→D, and D→A. 2. Calculate the net ΔU, W, and Q, for the complete cycle. Related conceptual questions: A. In which step(s) is work done by the system? B. In which step(s) does energy as heat flow out of the system? C. What part (region) of the PV plot represents the net work for the cycle? D. What do the signs of the net ΔU, W, and Q, for the complete cycle, indicate?