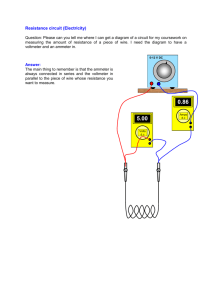
physical-sciences-grade-10-term-2
advertisement

Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT Physical Sciences WEEK 14 LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES TOPIC Physical and Chemical change (chemical change) - Time: 60 minutes Lesson 1&2 DATE COMPLETED: At the end of this lesson learners should be able to: Describe what happens to matter when it undergoes a chemical change. List examples of chemical changes that matter undergoes. The following results will be the outcome of this lesson: To re-enforce the concepts of physical and chemical change. The learners being able to describe and explain the chemical changes that elements and compounds undergo. TEACHING and LEARNING ACTIVITIES 1. TEACHING METHOD/S USED IN THIS LESSON: Question and Answer; Narrative 2. LESSON DEVELOPMENT 2.1 Introduction Introduce the lesson with an exciting demonstration/ experiment OR explain an example where the lesson is applied to life in general. e.g. Half fill a test tube with HYDROGEN PEROXIDE liquid, wait a few minutes and note what happens. Add a pinch of manganese dioxide and note what happens. Learners observe the experiment/ record their results and observations/ listen and follow demonstration. [20 min.] PRE-KNOWLEDGE A basic understanding of : Atoms; molecules ; compounds and elements Matter and the different phases in which it is found in. Chemical changes and how to explain what happens in a chemical change. EDUCATOR tests pre-knowledge by using the question and answer method as indicated in the baseline assessment. BASELINE ASSESSMENT: QUESTIONS/ ACTIVITY [10 min.] ● What is matter? What are the components of matter? What are the different phases of matter? ● What are atoms? Give examples. Term 2 Page 1 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans ● What are molecules? Give examples. ● What is a physical change and list examples of physical changes. ● What is a chemical change and give examples of chemical changes. 2.2 Main Body (Lesson presentation) Educator starts lesson off with an exciting demonstration as mentioned in the introduction and explains the relevant concepts in terms of a chemical change. [20 min.] Matter is all around us and it undergoes changes all the time, these changes can be classified as PHYSICAL CHANGES OR CHEMICAL CHANGES. The CONCEPTS of PHYSICAL CHANGE and CHEMICAL CHANGE is shown in the table below, educator engages learners in a discussion of the different aspects of physical and chemical change as mentioned in the table to re-enforce the concepts. physical changes chemical changes common signs that a chemical change has occurred are: common signs that a physical change has occurred are: 1. Production of gas bubbles 2. Change in the way something smells 3. A release of energy such as a flash or a sound (like a firecracker) 4. A precipitate forms (two liquids mixed together form a solid and a liquid) 1. change in the size 2. change in shape, 3. change in colour, or 4. Change in state/ phase of matter of a substance. 5. No new substance is produced. Examples of physical changes Examples of chemical changes • A grape when stepped on (changes shape) Blowing up a balloon (changes size and shape) • Liquid water turning to ice (changes state of matter) • Liquid water turning to steam (changes state of matter) • Mixing salt and sugar (changes the appearance, but you can still separate the mixture) • Mixing water and salt (changes the appearance, but you can still separate the mixture) Metal rusting (new substance formed) • Stomach digesting food (break down of food to new substances) • Plant carrying out photosynthesis (putting water and carbon dioxide together to make sugar) • Mixing baking soda and vinegar (makes a neutral liquid and a gas) Educator discusses the results of the experiment with the learners. The liquid hydrogen peroxide decomposes (breaks up) to form oxygen gas and liquid water (The atomic model the using ball and stick as well as space filling diagrams to show the reactants and products in this reaction). The MANGANESE DIOXIDE is a catalyst and speeds up the reaction. A CATALYST is a chemical substance that changes the speed of a reaction without getting used up in the process, at the end of the reaction the catalyst can be recovered. Eg. ENZYMES in our body help to speed up the digestive process. Term 2 Page 2 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Water 2 hydrogens : 1 oxygen Hydrogen Peroxide 2 hydrogens: 2 oxygens Oxygen gas 2 oxygens Educator engages learners in a question and answer session with regards the observations they have made. What do the products look like? How are they different from the original substances in terms of colour; phase; feel; smell etc.? Do the products have any of the properties of the original reactant, eg? Hydrogen peroxide is highly corrosive (burns your skin burning sensation), can be used as a bleaching agent (removes colour/ cleaning of metals) 3. Conclusion and Chalkboard summary Activity to Re-enforce lesson (Educator explains main concepts of the lesson and summarises points on chalkboard. (CHALKBOARD SUMMARY). [10 min.] During a Chemical change the particles themselves are changed in some way. There are greater energy changes that take place in a chemical change as compared to a physical change. The changes in energy are because energy is needed to break up bonds and then energy is given off when bonds are formed in the new products. It is very difficult to reverse a chemical change as can be seen from the fried egg that forms when the liquid egg is heated. In most chemical changes that take place the total mass remains of the reactants and products remain the same but the number of atoms and molecules change as shown below: Hydrogen Peroxide 2 hydrogens: 2 oxygens Water 2 hydrogens : 1 oxygen Oxygen gas 2 oxygens HOMEWORK QUESTIONS/ ACTIVITY (educator must give learners a few questions to answer at home by either writing them on the chalkboard or giving an exercise from the prescribed textbook) [10 min] Term 2 Page 3 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans i.e. 1. For each of the following say whether a CHEMICAL CHANGE or a PHYSICAL CHANGE occurs: 1.1 Melting candle wax. 1.2 Mixing sodium chloride and silver nitrate to form silver chloride and sodium nitrate. 1.3 Dissolving salt in water. 1.4 Melting a piece of plastic. 1.5 Burning a piece of paper. 2. Explain your answer for each of the changes that took place in the situations from 1.1 To 1.5. RESOURCES USED: Relevant apparatus (models/ atomic kits) and chemicals for practical demonstration; worksheet/ transparency for baseline assessment; relevant textbook/ notes eg (CAPS document pg. 35-37; chapter 12 from textbook PHYSICAL SCIENCES 10 pg. 109-113 (platinum series- Grayson; Harris; Mckenzie and Schreuder); grade 10 physical science version 1 caps pg. 192-196(Siyavula and volunteers). Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 4 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT Physical Sciences WEEK LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES 15 TOPIC Physical and Chemical change (conservation of matter) – Time: 60 minutes Lesson 1 DATE COMPLETED: At the end of this lesson learners should be able to: Illustrate the conservation of atoms and the non-conservation of molecules using atomic model diagrams (ball and stick and space filling) The following results will be the outcome of this lesson: To re-enforce the concepts of physical and chemical change. The learners being able to use atomic models and diagrams to describe and explain the chemical changes that elements and compounds undergo. TEACHING and LEARNING ACTIVITIES 1. TEACHING METHOD/S USED IN THIS LESSON: Question and Answer; Narrative 2. LESSON DEVELOPMENT 2.1 Introduction: Educator introduces the lesson with an exciting demonstration/ experiment OR explains an example where the lesson is applied to life in general. eg: Use the atomic model kit if available or use toothpicks and jelly tots to build the atomic/ molecular models for the reaction for the formation of the water molecule from the hydrogen molecule and the oxygen molecule and also build models for the decomposition of hydrogen peroxide reaction. Use these models to show the conservation of atoms and the non-conservation of molecules in a physical change. Learners observe the experiment/ record their results and observations/ listen and follow demonstration. [20 min.] PRE-KNOWLEDGE A basic understanding of : Atoms; molecules ; compounds and elements Chemical changes and how to explain what happens in a chemical change. EDUCATOR tests pre-knowledge by using the question and answer method as indicated in the baseline assessment. BASELINE ASSESSMENT: QUESTIONS/ ACTIVITY [10 min.] Term 2 Page 5 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans ● What is m matter? What are the components of o matter? What a are the different p phases of matter? ● What are e atoms? Give exa amples. ● What are e molecules? Give e examples. ● What is a physical change e and list exampless of physical chan nges. ● What is a chemical chang ge and give examples of chemical c changes. 2.2 Main Bo ody (Lesson prese entation) Educator e explains the LAW o of CONSERVATION of MATTER using tthe different mode els as well as diagrams and equatio ons. [20 min.] In a chemic cal reaction the TO OTAL NUMBER of ATOMS A remains CO ONSTANT BUT the e NUMBER of MOLE ECULES may CHAN NGE as seen below w in the reaction equations e for the formation o of water from hyd drogen gas and oxxygen gas and the e decomposition o of hydrogen perox xide. 2H2 2 Hydrogen P Peroxide molecule es = 4 hydrogen atoms + 4 oxygen n atoms = 8 atomss + O2 → 2H2O 2 Water molecules = 4 hydrogen atoms a + 2 oxygen n atom = 6 atoms 1 Oxygen molecule = 2 oxygen atoms The LAW W of CONSERVATIO ON of MATTER stattes that when elem ments and compo ounds bond to form m new products in n a chemical reac ction the number of o atoms of each type of elemen nt remains the sam me before and aftter the reaction. ANOTH HER 2 EXAMPLES SH HOWN BELOW to in ndicate that ATOM MS ARE CONSERVED in a CHEMICAL L REACTION. Term 2 Page 6 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans 3 molecules of hydrogen gas 6 atoms of hydrogen 2SO2 + 2 molecules of sulphur dioxide gas 2 atoms of sulphur and 4 atoms of oxygen 3H2 + N2 1 molecule of nitrogen gas 2 atoms of nitrogen O2 2NH3 2 molecules of ammonia 6 atoms of hydrogen and 2 atoms of nitrogen 2SO3 1 molecule of oxygen gas 2 atoms of oxygen 2 molecules of ammonia 2 atoms of sulphur and 6 atoms of oxygen CLOSED SYSTEM is when no outside factors (heat, other substances etc.) are added to the reaction to change the conditions to affect the reaction. Eg. Container must be closed when gases are involved. 3. Conclusion and Chalkboard summary Activity to Re-enforce lesson (Educator explains main concepts of the lesson and summarises points on chalkboard. (CHALKBOARD SUMMARY). [10 min.] During a Chemical change the particles themselves are changed in some ways. There are greater energy changes that take place in a chemical change as compared to a physical change. The changes in energy are because energy is needed to break up bonds and then energy is given off when bonds are formed in the new products. It is very difficult to reverse a chemical change as can be seen from the fried egg that forms when the liquid egg is heated. The total mass remains constant but the number of atoms and molecules change in most chemical reactions as shown above in the ball and stick and space filling models in the reaction with hydrogen peroxide. HOMEWORK QUESTIONS/ ACTIVITY (educator must give learners a few questions to answer at home by either writing them on the chalkboard or giving an exercise from the prescribed textbook) [20 min] i.e. 1. Copy the following diagrams in your books and complete the parts of the atoms and molecules that are missing. Term 2 Page 7 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans HYDROGEN GAS + OXYGEN GAS WATER 2. Draw the ball and stick models for the following chemical equations: Balance the following equations. All the reactants and products are shown. 2.1 Magnesium is burned in oxygen to give magnesium oxide: 2.2 Carbon and chlorine gas react to form carbon tetrachloride: 2.3 Potassium oxide is formed by burning potassium in oxygen: K + O2 K2O 2.4 Hydrogen reacts with chlorine gas to form hydrogen chloride: 3. Now balance the number of atoms and molecules on the product side and the reactants side of the equation. Draw the ball and stick models for the above balanced chemical equations: RESOURCES USED: Relevant apparatus (models/ atomic kits) and chemicals for practical demonstration; worksheet/ transparency for baseline assessment; relevant textbook/ notes eg (CAPS document pg. 35-37; chapter 12 from textbook PHYSICAL SCIENCES 10 pg. 112-116 (platinum series- Grayson; Harris; Mckenzie and Schreuder); grade 10 physical science version 1 caps pg. 197-201(Siyavula and volunteers). Term 2 Page 8 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 9 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT Physical Sciences WEEK LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES 15 TOPIC Physical and Chemical change (experiment) – Time: 60 minutes Lesson 3&4 DATE COMPLETED: At the end of this lesson learners should be able to: Illustrate the conservation of atoms and the non-conservation of molecules using an experiment. The following results will be the outcome of this lesson: To re-enforce the concepts of physical and chemical change. The learners being able to use atomic models and diagrams to describe and explain the chemical changes that elements and compounds undergo. TEACHING and LEARNING ACTIVITIES 1. TEACHING METHOD/S USED IN THIS LESSON: Question and Answer; Narrative 2. LESSON DEVELOPMENT 2.1 Introduction: Educator introduces the lesson with an exciting demonstration/ experiment OR explains an example where the lesson is applied to life in general. eg: Use the experiment between lead ii nitrate and sodium iodide OR sodium hydroxide and hydrochloric acid OR reacting Cal-C-Vita tablets with water to show the conservation of matter. The following apparatus and chemicals are needed: test tubes (4); 2 glass beakers; the respective chemicals; spatula (plastic spoons); 1 test tube stand; mass-meter; rubber stoppers. Learners observe the experiment/ record their results and observations/ listen and follow demonstration. [20 min.] PRE-KNOWLEDGE A basic understanding of : Atoms; molecules; compounds and elements. Chemical changes and how to explain what happens in a chemical change. EDUCATOR tests pre-knowledge by using the question and answer method as indicated in the baseline assessment. BASELINE ASSESSMENT : QUESTIONS/ ACTIVITY [10 min.] What is matter? What are the components of matter? What are the different phases of matter? Term 2 Page 10 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans What are atoms? Give examples. What are molecules? Give examples. What is a physical change and list examples of physical changes. What is a chemical change and give examples of chemical changes. 2.2 Main Body (Lesson presentation) Educator starts lesson off with an exciting demonstration about atoms and molecules using the experiment mentioned in the introduction and explains all the relevant concepts. [20 min.] METHOD for the experiment: Educator measures approximately 5 g of each substance OR uses 1/3 of a teaspoon of the lead ii nitrate and the sodium iodide powder in each test tube and fills the test tube up to the ¾ mark with water. The contents of the test tube are then shaken vigorously to dissolve the chemicals, use a rubber stopper to close the test tube before shaking it. If possible measure the mass of all the test tubes with their contents and record this mass. To a third test tube add ½ of the contents of each test tube to the third test tube and note your observations. Educator repeats experiment to make sure of results. After a few seconds a solid starts forming in the solution, this solid has a yellow colour and is the lead iodide that forms a precipitate because it in insoluble in water. Now measure the mass of each test tube after the reaction has taken place and compares it to the total mass before the reaction took place. Educator divides class into groups of 4 to 6 learners, depending on how many sets of apparatus he has. The learners then carry out the experiments in their groups and record their observations in the following table: [20 min.] mass of reactants and water mass of products and water REACTION 1 REACTION 2 3. Conclusion and Chalkboard summary Activity to Re-enforce lesson (Educator explains main concepts of the lesson and summarises points on chalkboard. (CHALKBOARD SUMMARY). [10 min.] Educator explains the LAW of CONSERVATION of MATTER using the BALANCED EQUATION as shown below. In a chemical reaction the TOTAL NUMBER of ATOMS remains CONSTANT before and after the reaction takes place. The number of REACTANT ATOMS is equal to the number of PRODUCT ATOMS. The TOTAL MASS at the start of the reaction is the same at the end of the reaction. Term 2 Page 11 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Pb(NO3)2 + 1 molecule of LEAD NITRATE 1 atom of lead + 2 atoms of nitrogen 6 atoms of oxygen 2NaI 2 molecules of sodium iodide = 2 atoms of sodium + 2 atoms of iodine 2 NaNO3 + 2 molecules of SODIUM NITRATE 2 atoms of sodium + 2 atoms of nitrogen + 6 atoms of oxygen PbI2 1 molecule of LEAD IODIDE 1 atom of lead + 2 atoms of iodine HOMEWORK QUESTIONS/ ACTIVITY (educator must give learners a few questions to answer at home by either writing them on the chalkboard or giving an exercise from the prescribed textbook) [10 min] 1. For each of the following definitions give the correct term: 1.1 a change that can be seen or felt where the particles are not broken up in any way. 1.2 The formation of new substances in a chemical reaction. 1.3 A reaction where a new product is formed from elements or smaller compounds. 2. Explain how a chemical change differs from a physical change, give two examples to support your explanation. RESOURCES USED: Relevant apparatus (models/ atomic kits) and chemicals for practical demonstration; worksheet/ transparency for baseline assessment; relevant textbook/ notes eg (CAPS document pg. 35-37; chapter 12 from textbook PHYSICAL SCIENCES 10 pg. 114-116 (platinum series- Grayson; Harris; Mckenzie and Schreuder); grade 10 physical science version 1 caps pg. 202-203(Siyavula and volunteers). Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 12 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT Physical Sciences WEEK LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES 16 TOPIC Physical and Chemical change (chemical change reaction equations) Time: 60 minutes Lesson 1 DATE COMPLETED: At the end of this lesson learners should be able to: Represent chemical changes using reaction equations. Translate word equations into symbol representation using the correct symbols for the elements and compounds. The following results will be the outcome of this lesson: To re-enforce the concepts of physical and chemical change. The learners being able to describe and explain the chemical changes that elements and compounds undergo. TEACHING and LEARNING ACTIVITIES 1. TEACHING METHOD/S USED IN THIS LESSON: Question and Answer; Narrative 2. LESSON DEVELOPMENT 2.1 Introduction: Educator introduces the lesson with an exciting demonstration/ experiment OR explains an example where the lesson is applied to life in general. eg: Use the atomic model kit if available or use toothpicks and jelly tots to build the atomic/ molecular models for the reaction for the formation of the water molecule from the hydrogen molecule and the oxygen molecule. Use these models to show the conservation of atoms and the non-conservation of molecules in a chemical change as well as the conservation of mass using symbols in a chemical equation. [20 min.] PRE-KNOWLEDGE A basic understanding of : Atoms; molecules ; compounds and elements Matter and the different phases in which it is found in. Chemical changes and how to explain what happens in a chemical change. EDUCATOR tests pre-knowledge by using the question and answer method as indicated in the baseline assessment. BASELINE ASSESSMENT: QUESTIONS/ ACTIVITY [10 min.] What is matter? What are the components of matter? What are the different phases of matter? Term 2 Page 13 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans What are atoms? Give examples. What are molecules? Give examples. What is a physical change and list examples of physical changes. What is a chemical change and give examples of chemical changes. 2.2 Main Body (Lesson presentation) Educator starts lesson by explaining the different components of a chemical equation. [20 min.] REACTANT A(S) + B(l) Directio n of PRODUCT C(g) + ∆H = + 50 J CHANGE IN ENERGY (Hproducts – Hreactants) PHASE D(aq) In a chemical reaction the REACTANTS are the chemicals that are put in a container at the start of the reaction. The reaction then takes place to form the PRODUCTS which is what is in the container after the reaction has taken place completely. A CHEMICAL EQUATION is a SYMBOL REPRESENTATION of the chemical reaction. The REACTANTS are always shown on the left hand side of the equation and the PRODUCTS are always on the right hand side. The ARROW that separates the reactants from the products shows the DIRECTION of the reaction. The LETTERS (s) indicates a SOLID; the letter (l) a LIQUID; the letter (g) a GAS and the letters (aq) AQUEOUS..... these letters show the PHASE of the reactants and products in a chemical equation. In some reactions the ΔH is shown which means the CHANGE in ENERGY of a particular reaction, the amount of energy that is given off or taken in a chemical reaction. If the ΔH value is POSITIVE then the reaction is an ENDOTHERMIC REACTION, if it is a NEGATIVE value then the reaction is an EXOTHERMIC reaction. ENDOTHERMIC REACTION is a reaction in which energy is absorbed from the surrounding for the reaction to take place, the temperature of the reaction mixture decreases as the reaction takes place. EXOTHERMIC REACTION is a reaction in which energy is given off to the surrounding, the temperature of the reaction mixture increases as the reaction takes place The equations below show word equations which is then translated into chemical equations using the correct symbols for the elements and compounds.(the equations are not balanced) 1. HYDROGEN GAS H2(g) + + OXYGEN GAS O2 (g) WATER H2 O (l) Term 2 Page 14 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans 2. HYDROGEN GAS + + H2 NITROGEN GAS N2 AMMONIA NH3 1. 3. SULPHUR DIOXIDE SO2 + + OXYGEN O2 SULPHUR TRIOXIDE SO3 3. Conclusion and Chalkboard summary Activity to Re-enforce lesson (Educator explains main concepts of the lesson and summarises points on chalkboard. (CHALKBOARD SUMMARY). [10 min.] Educator discusses the writing of formulae with learners to re-enforce this skill. The following exercise is attempted by learners and then educator discusses answers on the board using ionic equations and charges. Learners to write down the chemical formula of the following compounds: 1. Sodium chloride 2. Magnesium fluoride 3. Potassium oxide 4. Aluminium oxide 5. Zinc nitrate 6. Aluminium sulphate 7. Iron iii chloride 8. Potassium dichromate 9. Ammonium phosphate 10. Sulphur iv oxide HOMEWORK QUESTIONS/ ACTIVITY (educator must give learners a few questions to answer at home by either writing them on the chalkboard or giving an exercise from the prescribed textbook) [10 min] i.e. Write down the correct chemical name for each of the following: 1. SO2 2. KMnO4 3. (NH4)2SO4 4. Fe3(PO4)2 5. KClO3 RESOURCES USED: Relevant apparatus (models/ atomic kits) and chemicals for practical demonstration; worksheet/ transparency for baseline assessment; relevant textbook/ notes eg (CAPS document pg. 35-37; chapter 12 from textbook PHYSICAL SCIENCES 10 pg. 118-119 (platinum series- Grayson; Harris; Mckenzie and Schreuder); grade 10 physical science version 1 caps pg. 206-207(Siyavula and volunteers). Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 15 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT Physical Sciences LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES WEEK 16 TOPIC Physical and Chemical change (balanced reaction equations using atomic models) – Time: 60 minutes lesson 2 DATE COMPLETED: At the end of this lesson learners should be able to: Balance reaction equations using atomic models. Show the conservation of matter using balanced reaction equations. The following results will be the outcome of this lesson: To re-enforce the concepts of physical and chemical change. The learners being able to describe and explain the chemical changes that elements and compounds undergo. TEACHING and LEARNING ACTIVITIES 1. TEACHING METHOD/S USED IN THIS LESSON: Question and Answer; Narrative 2. LESSON DEVELOPMENT 2.1 Introduction: Educator introduces the lesson with an exciting demonstration/ experiment OR explain an example where the lesson is applied to life in general. eg: Use the atomic model kit if available or use toothpicks and jelly tots to build the atomic/ molecular models for the reaction for the formation of the water molecule from the hydrogen molecule and the oxygen molecule . Use these models to show the conservation of atoms and the non-conservation of molecules in a chemical change. [20 min.] PRE-KNOWLEDGE A basic understanding of : Atoms; molecules ; compounds and elements The writing of chemical formulae using the table of ions. EDUCATOR tests pre-knowledge by using the question and answer method as indicated in the baseline assessment. BASELINE ASSESSMENT: QUESTIONS/ ACTIVITY [10 min.] List the components of a chemical equation. The writing of chemical formulae using the table of ions. Term 2 Page 16 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans 2.2 Main Bo ody (Lesson prese entation) Educator sttarts lesson by exp plaining the conservation of matter a and mass using th he atomic models and explains all other o relevant concepts. [20 min.] The LAW off CONSERVATION o of MATTER: In a chemical reaction the TOTAL NUMBE ER of ATOMS remains CONSTANT BUTT the NUMBER of MOLECULES M may CHANGE C as seen below b in the reaction eq quations for the fo ormation of water from hydrogen ga as and oxygen gas and the decomposition of hydrog gen peroxide. 1. Hydroge en Peroxide = 2 mo olecule 4 hydrog gen atoms + 4 oxyg gen atoms = 8 ato oms 2 Water mo olecule 4 hydrogen n atoms + 2 oxyge en atom = 6 atom ms 2 molecule es 8 atoms 1 Oxygen O molecule 2 oxxygen atoms 3 molecule es 8 atoms Educattor explains the prrocess for the BALA ANCING of chemical equations usin ng the chemical equation e for the ab bove model. 2 H2 O2 Hydrrogen Peroxide = 2 molecule 4 hyd drogen atoms + 4 oxygen atoms = 8 atoms 2 H2 O 2 Water molecule ogen atoms + 2 oxygen o atom = 6 atoms a 4 hydro O 1 Oxygen molecule 2 oxygen atoms 3 molec cules 8 atom ms 2 molec cules 8 ato oms Term 2 Page 17 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans 2. 3. Conclussion and Chalkboard summary Activity to R Re-enforce lesson (Educator expla ains main conceptts of the lesson and summarises poin nts on chalkboard d. (CHALKBOARD SUMMARY). S [10 min.] 1. Write the e unbalanced equ uation. ● Chemica al formulas of reactants are listed on n the left-hand side e of the equation.. ● Products are listed on the rright-hand side of the equation. ● Reactantts and products arre separated by putting p an arrow b between them to sshow the direction n of the reaction. Reactions R at equilibrium will have arrrows facing both directions. 2. Balance e the equation. ● Apply the e Law of Conserva ation of Matter to get g the same num mber of atoms of e every element on each side of the equation. e Tip: Startt by balancing an n element that app pears in only one re eactant and produ uct. ● Once one e element is balan nced, proceed to balance anotherr, and another, un ntil all elements are e balanced. ● Balance c chemical formulas by placing coeffficients in front of tthem. Do not add d subscripts, becau use this will change the formulas. The number in FRON NT of a compound d applies to ALL the ELE EMENTS in the com mpound. 3. Indicate e the states of mattter of the reactantts and products. ● Use (g) fo or gaseous substan nces; (s) for solids; (l) for liquids; (aq) for species in solu ution in water. ● Write the state of matter im mmediately followiing the formula of the substance it d describes. RK QUESTIONS/ AC CTIVITY (educator must give learnerss a few questions tto answer at home e by either writing them on the chalkboard or giving an a exercise from the t HOMEWOR prescribed textbook) [10 min n] Balance ea ach of the followin ng chemical equa ations, using marbles of different colours to represent the t different atom ms and sticking the e marbles together with prestik to ma ake molecules. Now balance the e number of atoms on both sides of the reaction as sh hown below in the e equations. 1. Cl2 (g) + H2(g) HCll(g) 2. Br2 (g) + H2(g) HBrr(g) 3. P (s) + C Cl2(g) PCl3(g) Term 2 Page 18 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans RESOURCES USED: Relevant apparatus (models/ atomic kits) and chemicals for practical demonstration; worksheet/ transparency for baseline assessment; relevant textbook/ notes eg (CAPS document pg. 35-37; chapter 12 from textbook PHYSICAL SCIENCES 10 pg. 118-119 (platinum series- Grayson; Harris; Mckenzie and Schreuder); grade 10 physical science version 1 caps pg. 209-214(Siyavula and volunteers). Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 19 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT Physical Sciences LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES WEEK 16 TOPIC Physical and Chemical change(balanced reaction equations) - Time: 60 minutes Lesson 3 DATE COMPLETED: At the end of this lesson learners should be able to: Write reaction equations from word equations and balance them. Show the conservation of matter using balanced reaction equations. The following results will be the outcome of this lesson: To re-enforce the concepts of physical and chemical change. The learners being able to describe and explain the chemical changes that elements and compounds undergo. TEACHING and LEARNING ACTIVITIES 1. TEACHING METHOD/S USED IN THIS LESSON: Question and Answer; Narrative 2. LESSON DEVELOPMENT 2.1 Introduction: Educator introduces the lesson with an exciting demonstration/ experiment OR explain an example where the lesson is applied to life in general. eg: Use the atomic model kit if available or use toothpicks and jelly tots to build the atomic/ molecular models for the reaction for the formation of the water molecule from the hydrogen molecule and the oxygen molecule . Use these models to show the conservation of atoms and the non-conservation of molecules in a chemical change using symbols in a chemical equation, the chemical equation must match the number of atoms and molecules in the atomic models being built. Use these models to show how to balance an equation. [20 PRE-KNOWLEDGE A basic understanding of : Atoms; molecules ; compounds and elements The components of a chemical equation. EDUCATOR tests pre-knowledge by using the question and answer method as indicated in the baseline assessment. BASELINE ASSESSMENT : QUESTIONS/ ACTIVITY [10 min.] List the components of a chemical equation What are atoms? Give examples. Term 2 Page 20 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans What a are molecules? Giv ve examples. 2.2 Main Bo ody (Lesson prese entation) Educator sttarts lesson by exp plaining the writing g of chemical equations from word equation and sho owing learners how w to write the form mula of compound ds and then explaiining the balancing of equations as sh hown in the three examples e done below. [20 min.] 1. HYDRO OGEN GAS + H2 (g) + OXYGEN GA AS WATER O2 (g) H2 O (l) Since there are 2 OXYGEN atoms on the reac ctant side and only 1 on the produc ct side the equatio on is not balanced d, to balance it pu ut a 2 in front of th he H2O and the OXYGEN e balanced but th his 2 also increasess the HYDROGEN a atoms to 4 on the product side, therefore a 2 must go o in front of the H2 on the reactant side to give a total of 4 H atoms are atoms on both sides of the equation. An equ uation can only be e balanced by CH HANGING the NUM MBER in FRONT of an a ELEMENT or CO OMPOUND in an eq quation as shown below. HYDROGE EN GAS + 2 H2 (g) + OXYGEN GAS O2 (g) 2 H2O(l) 2. Iron re eacts with sulphurr to form iron ii sulp phide Fe (s) + S (s) FeS(s) Since 1 a atom of iron reactss with 1 atom of su ulphur and the fina al product has 1 a atom of iron and 1 atom of sulphur the equation is balanced, it does no ot need any coefficients to balance it 3. Aluminium metal reactts with oxygen gass to form aluminium m oxide Al(s) + O2 (g) Al2O3(s) Since the e product has 3 Oxxygen atoms, Whe en balancing equa ations try to make e the ODD NUMBER R of atoms into an n EVEN NUMBER an nd then it becomess easier to balance e the rest of the atoms, to make the e OXYGENS into an even number it has to be multiplie ed by 2, this INCRE EASES the OXYGEN N ATOMS on the product side e to 6, BUT a 2 in frront of the ALUMIN NIUM OXIDE AFFEC CTS the aluminium as well as the oxy ygens as follows: Al(s) + O2 (g) 2 Al2O3(s) this now increases the ALUM MINIUM ATOMS to o 4 on the PRODUC CT SIDE, therefore you need 4 Aluminium atoms on the e REACTANT SIDE 4 Al(s) + O2 (g) 2 Al2O3(s) Term 2 Page 21 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans 4. BUT no ow you need to MU ULTIPLY OXYGEN ATOMS A on the REA ACTANT SIDE by 3 tto balance the 6 OXYGENS O on the PRODUCT P SIDE 4 Al(s) + 3 O2 (g) 2A Al2O3(s) plete equation is n now balanced, ch heck: The comp 4 Al atom ms + 6 O atoms on reactant side eactant side 4 Al atoms + 6 O atoms on re Educator e explains the processs for the BALANC CING of chemical e equations: Educa ator uses the follow wing examples to show s balancing of chemical equations and the consservation of matter and d mass. 3. Conclussion and Chalkboard summary Activity to R Re-enforce lesson (Educator expla ains main conceptts of the lesson and summarises poin nts on chalkboard d. (CHALKBOARD SUMMARY). S [10 min.] The followin ng example is don ne on the board to o re-enforce the skkills in the writing a and balancing of chemical c equatio ons Worked Exa ample Problem Tin oxide is heated with hydro ogen gas to form tin metal and watter vapour. Write tthe balanced equ uation that describ bes this reaction. 1. Write tthe unbalanced e equation. SnO2 + H2 → Sn + H2O 2. Balanc ce the equation. Look a at the equation an nd see which elem ments are not bala anced. In this case e, there are two ox xygen atoms on th he left-hand side of o the equation and only one on the righthand side. Correct this b by putting a coeffficient of 2 in frontt of water: SnO2 + H2 → Sn + 2 H2O This pu uts the hydrogen a atoms out of balance. Now there are two hydrogen a atoms on the left and a four hydrogen atoms on the rig ght. To get four hyd drogen atoms on the right, add a coefficient of 2 fo or the hydrogen gas. g Remember, coefficients are mu ultipliers, so if we write w 2 H2O it denotes 2x2=4 hydroge en atoms and 2x1= =2 oxygen atoms. SnO2 + 2 H2 → Sn + 2 H2O The eq quation is now balanced. Be sure to o double-check yo our math! Each sid de of the equation n has 1 atom of Sn n, 2 atoms of O, an nd 4 atoms of H. 3. Indica ate the physical sta ates of the reactants and products. To do this, you need to be familiar with th he properties of va arious compoundss or you need to be told what the phases are for the chemicals c in the re eaction. Oxides are solids, hydrogen forms a diato omic gas, tin is a so olid, and the term 'water vapor' indicates that water is i in the gas phase e: SnO2(ss) + 2 H2(g) → Sn(ss) + 2 H2O(g) HOMEWOR RK QUESTIONS/ AC CTIVITY (educator must give learnerss a few questions tto answer at home e by either writing them on the chalkboard or giving an a exercise from the t prescribed textbook) [20 min n] Term 2 Page 22 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Balance the following equations: Balance the following chemical equations showing the conservation of atoms. 1. Fe + H2S04 Fe2(SO4)3 + H2 H2O + CO2 K3PO4 + H2O 2. C2H6 + O2 3. KOH + H3PO4 4. SnO2 + H2 Sn + H2O 5. NH3 + O2 NO + H2O RESOURCES USED: Relevant apparatus (models/ atomic kits) and chemicals for practical demonstration; worksheet/ transparency for baseline assessment; relevant textbook/ notes eg (CAPS document pg. 35-37; chapter 12 from textbook PHYSICAL SCIENCES 10 pg. 120-121 (platinum series- Grayson; Harris; Mckenzie and Schreuder); grade 10 physical science version 1 caps pg. 209-214(Siyavula and volunteers). Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 23 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT Physical Sciences LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES WEEK 16 TOPIC Physical and Chemical change (interpret balanced equations in terms of mass) – Time: 60 minutes Lesson 4 DATE COMPLETED: At the end of this lesson learners should be able to: Interpret balanced reaction equations in terms of the conservation of mass. Show the conservation of matter using balanced reaction equations. The following results will be the outcome of this lesson: To re-enforce the concepts of chemical change. The learners being able to explain balanced chemical equations in terms of mass. TEACHING and LEARNING ACTIVITIES 1. TEACHING METHOD/S USED IN THIS LESSON: Question and Answer; Narrative 2. LESSON DEVELOPMENT 2.1 Introduction: Educator introduces the lesson with an exciting demonstration/ experiment OR explain an example where the lesson is applied to life in general. eg: Use the atomic model kit if available or use toothpicks and jelly tots to build the atomic/ molecular models for the reaction for the formation of the water molecule from the hydrogen molecule and the oxygen molecule . Use these models to show the conservation of atoms and the non-conservation of molecules in a chemical change as well as the conservation of mass using symbols in a chemical equation. [20 min.] PRE-KNOWLEDGE : A basic understanding of : How to write formulae of compounds and elements The components of a chemical equation. Calculating relative atomic mass of elements and compounds from the periodic table. EDUCATOR tests pre-knowledge by using the question and answer method as indicated in the baseline assessment. BASELINE ASSESSMENT : QUESTIONS/ ACTIVITY [10 min.] List the components of a chemical equation Calculating relative atomic mass of elements and compounds using the periodic table. Term 2 Page 24 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans 2.2 Main Bo ody (Lesson prese entation) Educattor starts lesson by y writing the chemical equations from word equationss and showing lea arners how to write e the formula of co ompounds . The ed ducator then exp plains the balanc cing of equations u using the law of co onservation of ma ass. The relative atomic of each elem ment in the reacta ants and the products are obtained d from the periodic c table as shown in the three exam mples done below.. [20 min.] Law of conversation of m mass states that: Mass M can neither b be created nor desstroyed during a chemical c reaction n. During a chemic cal reaction total mass m of products iss equal to the tota al mass of reactan nts. In a chemic cal equation then n, the mass of the reactants r must be e equal to the masss of the products. In order to make sure that this is the e case, the number of atoms of eac ch element in the reacttants must be equ ual to the number of atoms of those e same elements in n the products. So ome examples are e shown below: Example 1: Fe + S Reactants : Atomic mass of reactants = 56 + 32 = 88 g → FeS Num mber of atoms of each element in the t reactants: (1 × Fe) and (1 ( Product: Atomic mass of pro oduct = 56 + 32 = 88 g Number o of atoms of each e element in the products: (1 × Fe) and (1 Since the number of atoms o of each element is the same in the re eactants and in th he products, we sa ay that the equatio on is balanced. × × S) S) Example 2: H2 + O2 → H2O Reactants: Atomic mass of re eactants = (1 + 1) + (16 + 16) = 34 g Number of atom ms of each elemen nt in the reactantss: (2 × H and (2 H) × O) Product: Atomic mass of pro oduct = (1 + 1 + 16 6) = 18 g Number o of atoms of each e element in the pro oducts: (2 × H) and (1 × O) Since the to otal atomic mass o of the reactants and a the products iss not the same an nd since there are more oxygen ato oms in the reactan nts than there are in i the product, the e equation is not balan nced. The equatio on can be balance ed as follows: 2 H2 + O2 → 2 H2O Mass of Rea actants in balance ed equation: Atom mic mass of reacttants = 2(1 + 1) + (1 o each element in n the reactants: (2 x 2 x H = 4 H) and d (2 X O = 2 16 + 16) = 36 g Number of atoms of O atoms) Term 2 Page 25 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Mass of Pro oducts in balanced d equation: Atom mic mass of produc ct = (1 + 1 + 16) = 1 18 x 2 = 36 g Num mber of atoms of each element in th he products: (2 x 2 x H = 4 H) and (2 X O = 2 O atoms) Example 3: NaOH + HC Cl → NaCl + H H2O Reactants : Atomic mass of re eactants = (23 + 16 + 1) + (1 + 35.5) = 76.5 g Number of atoms of each element in the rea actants: (1 × Na) + (1 × O) + (2 × H) + (1 × Cl) Products : A Atomic mass of pro oducts = (23 + 35.5) + (1 + 1 + 16) = 76.5 g Number of atoms of each element in the prod ducts: (1 × Na) + (1 1 × O) + (2 × H) + (1 × Cl) Since the number of atoms o of each element is the same in the re eactants and in th he products, we sa ay that the equatio on is balanced. 3. Conclussion and Chalkboard summary Activity to R Re-enforce lesson (Educator expla ains main conceptts of the lesson and summarises poin nts on chalkboard d. (CHALKBOARD SUMMARY). S [10 min.] The followin ng example is don ne on the board to o re-enforce the skkills in the writing a and balancing of chemical c equatio ons Worked Exa ample Problem Methane re eacts with oxygen n to form carbon dioxide d and waterr. Write the balanc ced equation that describes this rea action. 1. Write tthe unbalanced e equation. CH4(g) + O2(g) → CO2(g) + H2O(l) 2. Balanc ce the equation. Look a at the equation an nd see which elem ments are not bala anced. In this case e, there are two ox xygen atoms on th he left-hand side of o the equation and three on the rig ght-hand side. C Correct this by puttting a coefficient of 2 in front of wa ater and a 2 in fron nt of the oxygen: CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) This pu uts the hydrogen a atoms out of balance. Now there are four hydrogen atoms on the left and four hydrogen atoms on the rig ght. To get four hydrogen atoms on the right, add a coefficient of 2 fo or the water. Rem member, coefficien nts are multipliers, so if we write 2 H2O it denotes 2x2=4 4 hydrogen atomss and 2x1=2 oxyge en atoms. The eq quation is now balanced. Be sure to o double-check yo our math! Reactants : Atomic mass of re eactants = (12 + 4 x 1) + (2 x 16 x 2) = 80 g Number off atoms of each ellement in the reac ctants: (1 × C) + (4 4 × H) + (4 × O) Products : A Atomic mass of pro oducts = (12 + 2 x 16) + (2 x 18) = 80 0 g Number of atoms of each eleme ent in the productss: (1 × C) + (4 × O)) + (2 × H) HOMEWOR RK QUESTIONS/ AC CTIVITY (educator must give learnerss a few questions tto answer at home e by either writing them on the chalkboard or giving an a exercise from the t prescribed textbook) [20 min n] Term 2 Page 26 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Balance the following chemical equations showing the conservation of mass of reactants and products. Fe2(SO4)3 + 1. Fe + H2S04 2. C2H6 + O2 3. KOH + H3PO4 4. SnO2 + 5. NH3 + H2 H2O + CO2 K3PO4 + H2O H2 Sn + H2O O2 NO + H2O RESOURCES USED: Relevant apparatus (models/ atomic kits) and chemicals for practical demonstration; worksheet/ transparency for baseline assessment; relevant textbook/ notes eg (CAPS document pg. 35-37; chapter 12 from textbook PHYSICAL SCIENCES 10 pg. 122-123 (platinum series- Grayson; Harris; Mckenzie and Schreuder); grade 10 physical science version 1 caps pg. 209-214(Siyavula and volunteers). Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 27 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES WEEK 17 122222 TOPIC Lesson 1 1. Learners will be taught and learn the following concepts: Classification of materials as magnetic or non-magnetic Daily applications of magnets Magnetic field of a permanent magnet 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Test and classify materials as magnetic or non-magnetic Give examples of materials that are magnetic and materials that are non-magnetic Describe the daily applications of magnets Explain the magnetic field of a permanent magnet 1. Teaching methods Observation, Investigative and Question and answer 2. Lesson development: 2.1 Introduction a. Pre-knowledge required. The force of attraction and force of repulsion Classifying materials as metals or non-metals b. Baseline assessment Refer to learner activities Do corrections on the board explaining and clarifying misconceptions. 2.2 Main Body (Lesson presentation) If enough magnets are available, learners should do this investigation themselves materials – Time: 60 minutes DATE COMPLETED: TEACHER ACTIVITIES c. Magnetic and non-magnetic Put the iron nail and lower the bar magnet nearer the magnet Learners will record their observations Repeat the same steps for all materials listed on the resources column and record the results on the structured form. e.g. LEARNER ACTIVITIES 1. Baseline Activity 1 TIMING Baseline: 5 min 1.1 Define the term force 1.2 Name two types of contact forces that can be exerted on an object. 1.3 What happens to an Iron nail as the magnet is passed nearer the nail? 1.4 What happens to an plastic comb as the magnet is passed nearer the comb? 2.2 Demonstration Activity 2 2.1 Classify the following as magnetic or non magnetic. Use the table on the teacher’s presentation column. RESOURCES NEEDED Check the available resource like textbook, question papers etc Demonstration 25 min • Bar magnet • Iron nails • Copper pieces Answering • Zinc plate 10 min • Plastic comb • Glass Term 2 Page 28 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans materials magnetic Non-magnetic Iron nails Copper pieces Zinc plate Plastic comb glass Wood and etc. Learners will provide more examples of magnetic and non-magnetic materials Place an iron nail on the table and pass a bar magnet 10 cm above the nail and ask learners to record their observations Learners try to explain their observations and the teacher clarifies the concept The teacher uses learners observations to explain the concept of magnetic field. Some of materials of which the magnet is made are named in class. e.g. cobalt, nickel, iron and its ores (magnetite and hematite) Learners list some daily applications of magnets .e.g. magnet strips on fridge doors, speakers, telephone etc 2.2 Other than examples given above, give two examples of magnetic substances and examples of nonmagnetic substances. 2.3 Name and describe three different applications of the magnets. • Graphite • aluminium Corrections : 10 min Conclusion : 5 minutes Learner’s questions 5 min 2.3 Conclusion • Wood Summarise the lesson considering definition of a magnetic field, classification of materials as magnetic or non-magnetic, the earth’s magnetic field and the electric field. Applications and importance of magnets need to be emphasised at this stage Homework : 30 min Term 2 Page 29 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 30 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 17 122222 TOPIC MAGNETIC FIELD OF A PERMANENT MAGNET – Time: 60 minutes Lesson 2 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: Magnet as an object with two poles Attraction and repulsion of magnetic poles Magnetic field pattern around a permanent magnet LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Describe a magnet as an object with two opposite poles Predict the behaviour of the magnets when they are brought close together Sketch the magnetic field patterns, showing the shape, size and direction of magnetic field TEACHER ACTIVITIES 1. Teaching methods Demonstration, Observation, & Question and answer 2. Lesson development: 2.1 Introduction a. Pre-knowledge required. Magnetic and non-magnetic materials Attraction and repulsion forces as a result of magnetic field b. Baseline assessment Refer to learner activities c. Do corrections on the board explaining and clarifying misconceptions. LEARNER ACTIVITIES 1. Baseline Activity 1 2.2 Demonstration Activity 2 2.1 Draw the field line pattern around the bar magnet 2.2 Which direction will the magnetic field lines of a bar magnet be pointing? Term 2 Page 31 RESOURCES NEEDED Baseline: 5 min A4 paper size Bar magnet 1.1 Where will the magnetic object get attracted to the magnet? 1.2 Which objects may be attracted to the magnet? 1.3 Define magnetic field 1.4 What is the magnet consist of? Name three substances 2.2 Main Body (Lesson presentation) If enough magnets are available, learners should do this investigation themselves as groups Place an A4 size card paper on top of a bar magnet Evenly sprinkle iron filings over a sheet of card paper Tap the card lightly with your finger The iron filings now show the magnetic field pattern of a bar magnet Place the small compasses at various positions around the pattern to find direction of the field lines From the observation of the field lines and compass directions, draw the magnetic field lines around a bar magnet. TIMING Iron filling Several compasses Demonstration 30 min Answering questions 10 min © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans 2.3 Draw the field lines between unlike poles of the two bar magnets and explain whether the force experienced by the two magnets is attractive or repulsive 2.4 What will happen if a bar magnet is broken into two pieces right in the middle? Will it still have north pole and south pole or it will only be two separated poles? Explain Corrections : 5 min Repeat the same but with two bar magnets having their north poles facing each other and draw the field pattern Conclusion : 5 minutes Learner’s questions 5 min Repeat the steps above but with the south pole of one magnet facing the north pole of the second magnet and draw the field lines pattern Illustrate the attraction force shown by joined lines between magnets and repulsion force shown by bending lines between the south poles of two magnets facing each other Homework : 30 min 2.3 Conclusion Summarise the lesson considering shape size and direction of magnetic field lines. Indicate where the field is strong and where the field weak Term 2 Page 32 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 33 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES WEEK 17 122222 TOPIC EARTH’S MAGNETIC FIELD – TIME: 60 MINUTES Lesson 3 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: Direction of magnetic field of a bar magnet Comparison between Earth’s magnetic field and magnetic field of a bar magnet Magnetic poles and geographic poles 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Explain how the compass indicates the direction of a magnetic field Illustrate the difference between geographic poles and magnetic poles Name and describe phenomenon that are affected by earth’s magnetic field Discuss qualitatively how earth’s magnetic fields provide protection from solar winds TEACHER ACTIVITIES LEARNER ACTIVITIES 1. Teaching methods 1. Demonstration, Observation, & Question and answer Activity 1 2. Lesson development: 2.1 Introduction 1.1 Define magnetic field a. Pre-knowledge required. Magnetic field pattern of a permanent magnet Poles of a magnet b. Baseline assessment Refer to learner activities c. Do corrections on the board explaining and clarifying misconceptions. 2.2 Main Body (Lesson presentation) Demonstrate how the compass is used to find the direction of field lines around a bar magnet Use diagrams to indicate magnetic field lines around a bar magnet as in the previous lesson Compare the bar magnet with the earth as a big magnet, but be cautious that learners should not misunderstand you in terms of magnetic poles and geographic poles Baseline TIMING RESOURCES NEEDED Baseline: 5 min 1.2 Draw the magnetic field lines around a bar magnet and show the direction of the field 1.3 What are the two poles of a bar magnet? 2.2 Demonstration Activity 2 2.1 What is meant by the solar winds? 2.2 Describe briefly how the earth’s magnetic field provides protection from solar winds 2.3 How does Aurora Borealis (Northern lights) occur? Term 2 Page 34 Demonstration 30 min Answering questions 7 min © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans 2.4 Which molecules helps the animals to migrate in the earth’s magnetic field since they do not have compass and navigators like people? 2.5 Compare the magnetic field of the earth to the magnetic field of a bar magnet. Corrections : 8 min Conclusion : 5 min Demonstrate the difference between the geographic poles and the magnetic pole Illustrate that the magnetic poles also move about slightly over the time Use the earth’s magnetic field to explain the lights seen on the northern side due to objects sent off from the sun and the earths atmosphere - Northern lights(Aurora Borealis) Explain how animals migration is influenced by the earth’s magnetic field. ( Magnetite Fe3O4 ) was found on the heads of animals and flies). Assumption is that they use the earth’s magnetic field to navigate their journeys Learner’s questions 5 min Homework : 30 min 2.3 Conclusion Summarise the lesson explaining the earth as the big magnet. The earth behaves like a bar magnet and has poles. The difference between magnetic field of a bar magnet and earth’s magnetic field are explained. Explain the Aurora Borealis and the solar winds. Use geographical migration in certain seasons to emphasise ability of animals and flies to navigate using magnetic field Term 2 Page 35 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 36 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: LESSON OBJECTIVES WEEK 17 122222 TOPIC 2. Lesson development: 2.1 Introduction a. Pre-knowledge required. Positive and negative charges Attraction and repulsion forces b. Baseline assessment Refer to learner activities LEARNER ACTIVITIES 1. Baseline TIMING Balloon Activity 1 o Dry hair 1.1 What are the charges on a neutral Particle and how do they compare? o Plastic ruler o Small pieces of 1.2 Name the force that exist between unlike charges o 1.3 Define an insulator o Electroscope o V.d. Graaff Baseline: 5 min paper Demonstration 2.2 Demonstration Activity 2 A learner rubs two substances, plastic and wool together. 2.1 Which particles will move between the plastic and wool? Term 2 Page 37 Running water from tap Do corrections on the board explaining and clarifying misconceptions. Start the lesson with demonstration to make the lesson interesting Rub a plastic ball pen on the dry hair and draw it nearer small pieces of paper. Ask learner to explain their observation (Only if teaching in the laboratory) Rub a plastic ruler and bring it closer to thin running tap water. Learners will explain reason for water to bend as it approaches ruler A balloon is rubbed against dry hair, and brought closer to smooth flowing water. Learners observe and illustrate their observation Define static electricity and electrification, give examples and explain why it occurs RESOURCES NEEDED o 2.2 Main Body (Lesson presentation) 4 1. Learners will be taught and learn the following concepts: Charges of a particle of an object Charging insulators by contact (tribo-electric charging) 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Name two particles found in an atom Identify number of protons and electrons in all neutral objects Determine the excess electrons on negatively charged particles and electrons deficiency on a positively charged particles Describe how an insulator may be charged by contact, and the type of charge they acquire 1. Teaching methods Demonstration, Observation, & Question and answer Lesson DATE COMPLETED: TEACHER ACTIVITIES c. ELECTROSTATICS – TIME: 60 MINUTES generator 30 min Answering questions 7 min © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Use electrons and protons to illustrate a neutral atom will have equal number of protons and electrons Explain that rubbing a neutral object may result in transfer of electrons, leading to an imbalance of protons and electrons, then an object becomes either positively or negatively charged Using an electroscope, demonstrate how a positively charged Perspex rod, on touching dome of electroscope , swings gold leaves away from each other. (A negatively charged PVC may be used) Should a V.d.Graaf generator be available, use it to demonstrate how the negatively charged long dry hair strands repel each other If time allows explain how a photocopier works, how lightning occurs , what should be done and what to avoid during lightning or Spray painting ( better give a guided research task on this section) Define polarisation and how polarisation occurs 2.3 Conclusion 2.2 Refer to atomic structure to explain why the other particle in an atom does not get transferred between wool and plastic 2.3 Explain why the gold leaf of an electroscope rises when a charged object is brought nearer or touches the dome of electroscope Corrections : 8 min 2.4 Name two variables that can affect the strength of the force between two charged objects 2.5 How can each variable be changed to obtain a stronger force? Summarise the lesson considering shape size and direction of magnetic field lines around the bar magnet and use them to explain position and direction of earth’s poles and the magnetic poles. Illustrate the importance of magnetic field to both people and animals Conclusion: 5 min Learner’s questions 5 min Homework : 30 min Term 2 Page 38 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 39 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 17 TOPIC 122222 CONSERVATION OF CHARGE – TIME: 60 MINUTES Lesson 4 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: Principle of conservation of charge Application of principle of conservation of charge LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : State the principle of conservation of charge Calculate the charge after two identical spheres on insulating stands come into contact and separate again Identify that resulting charge on each sphere after contact is the same TEACHER ACTIVITIES 1. Teaching methods Demonstration & Question and answer 2. Lesson development: Introduction a. Pre-knowledge required. • Two charges usually acquired by an object • Algebraic sum of the charges b. Baseline assessment • Refer to learner activities c. Do corrections on the board explaining and clarifying misconceptions. LEARNER ACTIVITIES 1. Baseline TIMING RESOURCES NEEDED Baseline: 5 min Activity 1 1.1 What are the two charges that an object may acquire? 1.2 When do positive and negative charges develop? 1.3 What type of force will be experienced by two objects carrying like charges? 2.2 Main Body (Lesson presentation) Define an atom as a starting point State the particles an atom is consist of Identify the mass of each particle. ( protons, electrons and neutrons) Allocate the signs +ve and –ve to protons and electrons respectively. Give a reason why it is scientifically acceptable to allocate those signs in terms of their algebraic sum. Explain that neutrons has no charge Allocate the charge of 1.60 x10-19 C and give it the symbol e. Explain what makes the charge of an electron and proton differ Engage learners in discussion to explain when is an object electrically neutral Indicate to learners that the charge of an electron is the smallest amount of free charge discovered Provide symbol for charge and demonstrate how bigger charges and number of electrons can be calculated from the equation q = eN where N is an integer 2.2 Demonstration Activity 2 2.1 State the principle of conservation of charge 2.2 When is the charge quantized? 2.3 How many electrons must be removed from an electrically neutral silver dollar to give it a charge of + 2,4 micro-coulombs? Term 2 Page 40 Demonstration 35 min Answering questions 5 min © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Explain when is the charge said to be quantized Demonstration example : How many electrons are there in one coulomb of negative charge? Hint : Learners should always start by recopying the equation from the Data sheet provided in the exam. Teachers should provide learners with copies now Corrections: 5 min It is advisable for the learners to substitute without changing the subject of the formula. q = eN 1.00 = 1.6 x 10-19 x N N = 6,25 x 1018 Therefore there are 6,25 x 1018 electrons in 1 C of charge Conclusion: 5 min 2.3 Conclusion Learner’s questions Summarise the lesson explaining the earth as the big magnet. The earth behaves like a bar magnet and has poles. The difference between magnetic of a bar magnet and earth’s magnetic field are explained. Explain the Aurora Borealis and the solar winds. Use common migration in certain season to emphasise ability of animals and flies to navigate using magnetic field 5 min Homework: 30 min Term 2 Page 41 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 42 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT Physical Sciences LESSON SUMMARY FOR: DATE STARTED: WEEK 18 TOPIC Charge quantization – Time 60 minutes Lesson 1 DATE COMPLETED: The outcomes of the lesson are : At the end of the lesson learners should be able to : LESSON OBJECTIVES State the principle of quantization of charge Calculate the charge or number of electrons from the equation q = eN TEACHING and LEARNING ACTIVITIES 1. TEACHING METHOD/S USED IN THIS LESSON: Demonstration ; Question and answer method 2. LESSON DEVELOPMENT 2.1 Introduction a) PRE-KNOWLEDGE learners need understanding of the following: i) Pre-knowledge required. Two charges usually acquired by an object An atom and what is consisting an atom Charges on protons and electrons b) BASELINE ASSESSMENT (educator to design a worksheet/ transparency or write questions on the board [preferably a worksheet to save time] to gauge the learners memory of their relevant prior knowledge) [5 min] QUESTIONS for the BASELINE ASSESSMENT 1.1 Write the value of charge of an electron. 1.2 What are the particles consisting an atom? 1.3 What nature is the charge carried by an electron? c) Do corrections and clarify misconceptions 2.2 Main Body (Lesson presentation) [35 min] Define an atom as a starting point: A atom is the smallest particle of matter that can not be divided into simpler substances State the particles an atom is consist of : protons, electrons and neutrons) Identify the mass of each particle. ( protons, electrons and neutrons) Allocate the signs +ve and –ve to protons and electrons respectively. Give a reason why it is scientifically acceptable to allocate those signs in terms of their algebraic sum their algebraic sum is equal to zero. . Explain that neutrons has no charge. It is neither positive nor negative. It is naturally neutral. Allocate the charge of 1.60 x10-19 C and give it the symbol e. Explain what makes the charge of an electron and proton differ. Protons are much bigger compared to electrons. The ratio of electro: proton is approximately 1:1836 Engage learners in discussion to explain when is an object electrically neutral? Indicate to learners that the charge of an electron is the smallest amount of free charge discovered State the quantization of charge: every charge in the universe consists of integer multiples of the electron charge. Term 2 Page 43 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Provide symbol for charge and demonstrate how bigger charges and number of electrons can be calculated from the equation q = eN where N is an integer Explain when is the charge said to be quantized Demonstrate how to calculate number of electrons: example : How many electrons are there in one coulomb of negative charge? Hint : Learners should always start by recopying the equation from the Data sheet provided in the exam. Teachers should provide learners with copies now to start practicing. It is advisable for the learners to substitute without hanging the subject of the formula. q = eN 1.00 = 1.6 x 10-19 x N N = 6,25 x 1018 Therefore there are 6,25 x 1018 electrons in 1 C of charge Learners Activity [ 10 min] 2.1 State the principle of quantization of charge. 2.2 When is the charge quantized? Explain 2.3 Give reason why it is acceptable to allocate + and – on the protons and electrons respectively 2.4 Explain why electrically neutral substances have a charge of zero. .5 How many electrons must be removed from an electrically neutral silver dollar to give it a charge of + 2,4 micro-coulombs? Corrections [5 min] 3. Conclusion [5 min] Summarise the lesson explaining the earth as the big magnet. The earth behaves like a bar magnet and has poles. The difference between magnetic of a bar magnet and earth’s magnetic field are explained. Explain the Aurora Borealis and the solar winds. Use common migration in certain season to emphasise ability of animals and flies to navigate using magnetic field HOMEWORK QUESTIONS/ ACTIVITY (educator must give learners a few questions to answer at home by either writing them on the chalkboard or giving an exercise from the prescribed textbook) [30 min]. RESOURCES USED: A4 paper size, Bar magnet, Iron filling, Several compasses Worksheets Term 2 Page 44 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 45 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 18 TOPIC 122222 EMF, POTENTIAL DIFFERENCE(PD) TIME: 60 MINUTES Lesson 2 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: emf of a battery Potential difference across terminals of a battery Relationship between emf and potential difference of a battery LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Define an emf of a battery Define the potential difference across the ends of a conductor Identify the difference between emf and potential difference, and the unit of measurement for both Define the unit of measurement of potential difference (volt) TEACHER ACTIVITIES 1. Teaching methods Demonstration & Question and answer 2. Lesson development: 2.1 Introduction a. Pre-knowledge required. Connection of an ammeter in a circuit (in series) Connection of a voltmeter, across a battery, resistor, etc Symbols of components of a circuit b. Baseline assessment Refer to learner activities c. Do corrections on the board explaining and clarifying misconceptions. 2.2 Main Body (Lesson presentation) To make your lesson more interesting, provide learners with circuit components, mainly those quite relevant to the lesson. e.g. voltmeter, batteries, resistor and a switch. Learners will record the voltmeter reading for both an open switch and closed switch. (Voltmeter may be connected across a resistor or across a battery depending on time available). Lost volts may be introduced to simplify your explanation LEARNER ACTIVITIES 1. Baseline TIMING Baseline: 5 min Activity 1 1.1 What is the function of a voltmeter in a circuit? 1.2 How should a voltmeter be connected in circuit? RESOURCES NEEDED o Light bulbs o Resistors o Batteries o Ammeter o Voltmeter o Switch 1.3 In which unit is the potential difference measured? 1.4 What is the energy conversion that takes place in a battery? 1.5 Why is it that the ammeter can not be connected across a battery or a resistor in a circuit? Demonstration 2.2 Demonstration Activity 2 2.1 Define an emf Term 2 Page 46 30 min © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans From the results observed, define the potential difference in terms of potential energy per unit charge between the two points(The potential difference, V, between two points in a circuit is defined as the amount of work done, W, when one coulomb of charge passes from one point to the other point. The SI unit for potential difference is Volt, V and an emf in terms of maximum potential difference when no current flows Provide a unit for both quantities (volt) and define a volt Potential difference = energy transferred between two points Charge moving past the two points V = W Q Calculation demonstration may be done using: What is the potential difference of a light bulb if a charge of 17,5 C pass through it, and radiates 4 200 J of energy? Ask learners to always start by copying the formula from the data sheet V = W Q Substitute without changing the subject of the formula V = 4200 17,5 2.2 Calculate the potential difference across the terminals of a battery if a charge of 3 C gains 27 J of energy passing through the battery Answering questions 7 min 2.3 Although potential difference and emf are both measured in volts, they are not the same. Describe the difference between emf and voltage 2.4 2.5 Corrections: 8 min Conclusion: 5 min emphasise mark allocation here Learner’s questions 5 min Learners use their calculators (help them) to find the voltage from V = 4200 17,5 Homework: 30 min An answer without a unit is a wrong answer, practice that from class exercise to tests and assignments etc More questions may be added to activity two 2.3 Conclusion In conclusion, describe how the voltmeter can be connected in a circuit. Define emf, potential difference and the volt. Illustrate important calculation steps. Term 2 Page 47 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 48 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 18 122222 TOPIC RESISTANCE – TIME: 60 MINUTES Lesson 3 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: Current in a circuit Calculations based on I = Q ∆t LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Define electric current (I) State the unit in which current is measured Calculate the current that flows in a circuit or through certain component State the direction in which the current flows TEACHER ACTIVITIES 1. Teaching methods LEARNER ACTIVITIES 1. Baseline TIMING Baseline: 5 min RESOURCES NEEDED o Light bulbs Demonstration , Question and answer Activity 1 o Resistors 2. Lesson development: 2.1 Introduction 1.1 In which direction does current flow in a circuit? o Batteries o Ammeter a. Pre-knowledge required. Particles of an atom Relationship between current and resistance Conventional current b. Baseline assessment Refer to learner activities 1.2 Mention three particles of matter o Voltmeter o Switch c. Do corrections on the board explaining and clarifying misconceptions. 2.2 Main Body (Lesson presentation) Provide an ammeter (if possible the large scale that can be seen from any position in class) Ask learners to explain how ammeter should be 1.3 Which of the particles mentioned above is responsible for the low of electric current ? 1.4 Describe the relationship between current and resistance in a conductor and potential difference 1.5 How does direction of electric current differ from direction of flow of electrons? Demonstration: 30 min 2.2 Demonstration Answering questions: 10 min Term 2 Page 49 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Activity 2 2.1 Define current 2.2 In which SI unit is current measured? Define this SI unit of current 2.3 Describe how should an ammeter be connected in a circuit connected in a circuit Describe what the learners should consider if given ammeter to connect in a circuit. Learners are reminded of what the ammeter measures in a circuit(current) Use a simple circuit diagram to explain the direction of flow of charges in a circuit as opposed to direction of electric current 2.4 Calculate the current that flows when 100 C of charge pass through an ammeter in 5 seconds 2.5 A current of 10 A flows through a light bulb for an hour. How much charge flows through this light bulb in an hour? Corrections: 10 min Conclusion: 5 min Homework: 30 min Define electric current and write an equation from the definition : I = Q ∆t Describe each quantity and provide the unit of measurement for each NB : Please inform learners that it is scientifically unacceptable to use “sec” as unit of time and “amps” for the unit of current Assist learners to convert to SI units. Explain what each letter stands for, and demonstrate how to reach the required unit. e.g. If current is in amperes Term 2 Page 50 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans K H D Ampere (A) x 100 x 1000 d c m ÷ 10 x 10 ÷ 1000 ÷ 100 Introduce micro-, nano-, pico- at this stage and assign a scientific value for each Micro (µ) - x 10-6 Nano (n) - x 10-9 Pico (p) - x 10-12 For calculation purpose learners should follow the following steps: o Re-write equation as it appears on the information sheet o Substitute without changing subject of the formula 2.3 Conclusion Chalkboard / whiteboard summary concludes the lesson , considering the definition of current, ampere and correct approach on doing calculations Term 2 Page 51 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 52 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 18 122222 TOPIC VOLTAGE AND CURRENT MEASUREMENT Lesson TIME: 60 MINUTES 4 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: Connection of voltmeter and ammeter in a circuit Recording readings from both the ammeter and voltmeter Draw a circuit diagram LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Explain the correct connection of both an ammeter and voltmeter Accurately record readings from an ammeter and voltmeter Draw a circuit diagram with correct symbols for given components TEACHER ACTIVITIES 1. Teaching methods LEARNER ACTIVITIES 1. Baseline TIMING Baseline: 5 min RESOURCES NEEDED o Light bulbs Demonstration , Investigative & Question and answer Activity 1 o Resistors 2. Lesson development: 2.1 Introduction 1.1 What is an electric current? o Batteries o Ammeter o Voltmeter o Switch a. Pre-knowledge required. Definition of current and potential difference Symbols of components of a circuit b. Baseline assessment Refer to learner activities c. Do corrections on the board explaining and clarifying misconceptions. 1.2 Which instrument is used to measure current in a circuit? 1.3 What is the function of a voltmeter? 1.4 Draw the symbols for the following circuit components : A resistor, bulb, voltmeter, ammeter, a battery etc. 2.2 Main Body (Lesson presentation) Precaution: Because ammeters are sensitive, and to avoid damage, discuss the connection of ammeter and voltmeter before learners touch the apparatus. i.e. always ensure that the ammeter is connected in series, with the red connected to the side attached to positive of the battery and black connected to negative terminal from the battery starting with the biggest scale. Provide learners with clear instructions to set-up circuit that measures current through a resistor or light bulb. (If using demonstration method, learners should connect the circuit components themselves) 2.2 Demonstration Activity 2 2.1 Define an emf 2.2 Calculate the potential difference across the terminals of a battery if a charge of 3 C gains 27 J of energy passing through the battery Term 2 Page 53 Demonstration: 30 min Answering questions 7 min © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Learners should change the position of an ammeter and record their results Explain why an ammeter can not be connected in parallel with the resistor, battery or a light bulb Explain how the voltmeter should be connected in a circuit ( in parallel with resistor, battery etc. because it has higher resistance and no current passes through it) Allow learners to change the position of the voltmeter from battery to resistor or bulb and record the results Ask learners to draw a circuit diagram , you can add more components to 2.3 Although potential difference and emf are both measured in volts, they are not the same. Describe the difference between emf and voltage 2.4 Explain how the flow of charges differ with the flow of electrons in a circuit Corrections: 8 min 2.5 Conclusion: 5 min Learner’s questions 5 min the sketches below: Homework: 30 min 2.3 Conclusion Refer to the chalkboard/transparency summary, explaining how a voltmeter and an ammeter should be connected in a circuit. Review symbols and circuit diagrams for specific circuit. Term 2 Page 54 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 55 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 19 122222 TOPIC RESISTANCE – TIME: 60 MINUTES Lesson 1 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: Resistance and its unit Energy transformation in battery and other circuit components Application of resistors on daily lives LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Define resistance and ohm Illustrate the microscopic description of resistance in terms of electrons moving through the conductor Explain energy transformation in a battery and resistor TEACHER ACTIVITIES 1. Teaching methods Demonstration , Investigative & Question and answer 2. Lesson development: 2.1 Introduction a. Pre-knowledge required. Electric current and circuit diagrams Potential difference b. Baseline assessment Refer to learner activities c. Do corrections on the board explaining and clarifying misconceptions. 2.2 Main Body (Lesson presentation) Simple demonstration using an ammeter, voltmeter, wires and batteries can make the introduction of the lesson interesting. Connect the ammeter, voltmeter and the batteries together, then ask learners to record the results. Repeat the same, add the resistor and ask learners to record the results. Learners will compare the results observed without a resistor and the results observed with a resistor. Seek explanation of the drop in current as in the second observation LEARNER ACTIVITIES 1. Baseline TIMING Baseline: 5 min RESOURCES NEEDED o Light bulbs Activity 1 o Resistors 1.1 Define resistance o Batteries o Ammeter o Voltmeter o Switch 1.2 What is the SI unit of potential difference? 1.3 Describe the energy conversion that take place in : a) Radio speaker b) Light bulb c) Electric stove 1.4 Give the difference between emf and potential difference 1.5 Why can’t an ammeter be connected across the battery in a circuit? 2.2 Demonstration Demonstration: 30 min Activity 2 2.1 Define resistance Answering questions: 10 min Term 2 Page 56 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans o o o o Define resistance R as the property of matter that tends to oppose the flow of current in a conductor( If possible, show learners some of the common samples of the resistors) Define the unit of resistance(ohm) as one volt per ampere and show the ratio V I Give the microscopic description in terms of electrons moving through the conductor Identify the factors that influence the resistance of a conductor and explain how each factor affects the resistance of a metallic conductor ( If time allows, you may demonstrate to the learners. Otherwise summary is enough ) The longer the conductor, the higher is the resistance Resistance increase with an increase in Temperature Thicker conductors have lower resistance than thinner conductors of the same material Different materials will have different resistance. Nichrome (alloy of Nickel and Chromium) will have higher resistance than copper or aluminium Mention application of resistance in daily life, e.g. stove, heaters, geysers, electric iron, light bulbs, and etc. 2.2 In which SI unit is resistance measured? Define this SI unit 2.3 State the factors that influence resistance of a metallic conductor 2.4 What is the scientific name given to the ratio V ? I 2.5 A long nichrome wire has more resistance to current than a short one of the same thickness. Explain why. The heating effect of current is utilised in the electrical heating appliances such as electric iron, room heaters, water heaters, etc. All these heating appliances contain coils of high resistance wire made of nichrome alloy. When these appliances are connected to power supply by insulated copper wires then a large amount of heat is produced in the heating coils because they have high resistance, but a negligible heat is produced in the connecting wires because the wires have low resistance. Corrections: 10 min Conclusion: 5 min Homework: 30 min Term 2 Page 57 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans The heating effects of electric current is utilized in electric bulbs for producing light. When electric current passes through a thin high resistance tungsten filament of an electric bulb, the filament becomes white hot and emits light. An 'electric fuse' is an important application of the heating effect of current. When the current drawn in a domestic electric circuit increases beyond a certain value, the fuse wire gets over heated, melts and breaks the circuit. This prevents fire and damage to various electrical appliances. Explain why a battery in a circuit goes flat eventually by referring to energy transformation that take place in a battery and resistor. i.e. When the potential energy has been converted into other forms of energy and the difference in potential energy between the positive and the negative terminals of the battery is zero, the battery goes flat. 2.3 Conclusion Chalkboard / whiteboard summary concludes the lesson , considering the definition of resistance, ohm and the factors influencing resistance of a metallic conductor. Daily application of resistors should be stated to inform learners of link between Science in class and daily application (importance of resistors) of Science. Term 2 Page 58 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 59 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 19 TOPIC 122222 RESISTORS IN SERIES 2 – TIME: 60 MINUTES DATE COMPLETED: TIME : Lesson 3 60 MIN 1. Learners will be taught and learn the following concepts: Effect of resistors in series Total resistance in a circuit Potential difference across each resistor and total potential difference LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Measure resistance of each resistor and calculate the total resistance of the circuit Measure potential difference across of each resistor and calculate the total potential difference in a circuit Describe the effect of resistors connected in series TEACHER ACTIVITIES 1. Teaching methods Demonstration , Investigative & Question and answer LEARNER ACTIVITIES 1. Baseline TIMING Baseline: 5 min Activity 1 2. Lesson development: 2.1 Introduction a. Pre-knowledge required. Resistance, calculations and current Potential difference b. Baseline assessment Refer to learner activities c. Do corrections on the board explaining and clarifying misconceptions. 2.2 Main Body (Lesson presentation) From the previous summary : resistors in series are: o Potential dividers o Current is the same Total resistance increases and the circuit diagram, calculations to find resistance, current or potential difference can be done using R = V I RESOURCES NEEDED 1.1 Define resistance 1.2 How should ammeter and voltmeter be connected in a circuit 1.3 What is the relationship between current and resistance in a circuit? 1.3 Measure current on each resistor and record results 1.4 Measure potential difference on each one of the resistors and record the results o 3 Resistors o Batteries o 3 Ammeters o 4 Voltmeter o Switch 2.2 Demonstration Activity 2 2.1 What effect do resistors in series have on the total resistance of the circuit ? 2.2 A circuit consists of a 12 V battery connected across a single resistor. If the current in the circuit is 3 A, calculate the size of the resistor. (4Ω) 2.3 Two 5Ω resistors are connected in series with a 12 V battery. Determine: Term 2 Page 60 Demonstration: 30 min © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Provide learners with a question to demonstrate manner in which calculation(s) should be done in physical science. Example 1 The current through light bulb in the section of a circuit is 0,625 A whereas the voltmeter reading is 240 V. Calculate the resistance of the light bulb. 2.3 Conclusion (a) the potential difference across each resistor; and (b) the current flowing in the circuit. (6 V, 1.2 A) Answering questions 10 min 2.4 . Consider the following circuit and then answer the questions below. Chalkboard / whiteboard summary concludes the lesson , stating that resistors in series are: o Potential dividers o Current is the same o Total resistance increases Corrections :10 min Report writing skills can be practiced from time to time using demonstrations available. Conclusion: 5 min a. State the potential difference between X and Z. b. State the potential difference between X and Y. Homework: 30 min c. How much potential is left at Y In the circuit below, the reading on the ammeter is 3.2 A. Term 2 Page 61 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Determine: a. the reading on the voltmeter; b. the potential difference across the 40 resistor; and Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 62 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 19 TOPIC 122222 RESISTORS IN PARALLEL – TIME: 60 MINUTES Lesson 3 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: Effect of resistors in parallel Total resistance in a circuit Potential difference across each resistor and total potential difference LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Measure resistance of each resistor and calculate the total resistance of the circuit Measure potential difference across of each resistor and calculate the total potential difference in a circuit Describe the effect of resistors connected in parallel TEACHER ACTIVITIES 1. Teaching methods LEARNER ACTIVITIES 1. Baseline Demonstration , Investigative & Question and answer Activity 1 2. Lesson development: 2.1 Introduction 1.1 Define resistance 1.2 How should ammeter and voltmeter be connected in a circuit 1.3 What is the relationship between current and resistance in a circuit? 1.3 Measure current on each resistor and record results 1.4 Measure potential difference on each one of the resistors and record the results a. Pre-knowledge required. Resistance, calculations and current Potential difference b. Baseline assessment Refer to learner activities c. TIMING RESOURCES NEEDED Baseline: 5 min o 3 Resistors o Batteries o 3 Ammeters o 4 Voltmeter o Switch Do corrections on the board explaining and clarifying misconceptions. 2.2 Main Body (Lesson presentation) Divide learners into groups and provide in each group : 3 Resistors ( e.g. 1Ω, 3Ω, and 5Ω) 2.2 Demonstration Activity 2 Batteries 4 Ammeters 4 Voltmeter Switch, otherwise a simple class demonstration will do. Demonstration 2.1 What effect do resistors in series have on the total resistance of the circuit ? 30 min 2.2 Term 2 Page 63 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Depending on the time available, learners should be given chance to: write investigative question, hypothesis and identify variables. Assist them to identify dependent, independent and constant variables Connect three resistors in parallel to each other with the switch and batteries Connect an ammeter before each of the resistors, and the last ammeter closer to the batteries then record the current on each ammeter. e.g. Answering questions 2.3 10 min 2.4 2.5 Corrections: 10 min Conclusion: 5 min Homework: 30 min Current divides (branches) in parallel Draw the circuit diagram on the board and each learner copies it from the board with each of the ammeters marked on the board. e.g. A1, A2, and A3 Connect the voltmeters across each of the resistors and the fourth one should be connected across the batteries Learners will record the readings on each voltmeter. Total current is calculated from I1, I2, and I3 and the sum is compared to current in the ammeter closer to the batteries IT = I1 + I 2 + I3 2.3 Conclusion Chalkboard / whiteboard summary concludes the lesson , stating that resistors in parallel are: o Current dividers Term 2 Page 64 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans o Potential difference is the same o Total resistance decreases. Effective resistance of resistors in parallel is lower than the resistance in the smallest resistor Report writing skills can be practiced from time to time using demonstrations available. Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 65 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 19 TOPIC 122222 RESISTORS IN SERIES – TIME: 60 MINUTES Lesson 4 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: Effect of resistors in series Total resistance in a circuit Potential difference across each resistor and total potential difference LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Measure resistance of each resistor and calculate the total resistance of the circuit Measure potential difference across of each resistor and calculate the total potential difference in a circuit Describe the effect of resistors connected in series TEACHER ACTIVITIES 1. Teaching methods LEARNER ACTIVITIES 1. Baseline Demonstration , Investigative & Question and answer Activity 1 2. Lesson development: 2.1 Introduction 1.1 Define resistance 1.2 How should ammeter and voltmeter be connected in a circuit 1.3 What is the relationship between current and resistance in a circuit? 1.3 Measure current on each resistor and record results 1.4 Measure potential difference on each one of the resistors and record the results a. Pre-knowledge required. Resistance, calculations and current Potential difference b. Baseline assessment Refer to learner activities c. Do corrections on the board explaining and clarifying misconceptions. Main Body (Lesson presentation) Divide learners into groups and provide in each group : 3 Resistors ( e.g. 1Ω, 3Ω, and 5Ω) 4 Voltmeter Switch, otherwise a simple class demonstration will do. RESOURCES NEEDED Baseline: 5 min o 3 Resistors o Batteries o 3 Ammeters o 4 Voltmeter o Switch 2.2 Demonstration Activity 2 Batteries 4 Ammeters TIMING Demonstration: 30 min 2.1 What effect do resistors in series have on the total resistance of the circuit ? Answering questions: 10 min Term 2 Page 66 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Depending on the time available, learners should be given chance to: write investigative question, hypothesis and identify variables. Assist them to identify dependent, independent and constant variables Connect three resistors in series with the switch and batteries. Corrections: 10 min Conclusion: 5 min e.g. Connect an ammeter before each of the resistors, and the last ammeter closer to the batteries then record the current on each ammeter. Current is the same throughout the circuit Draw the circuit diagram on the board and each learner copies it from the board with each of the ammeters marked on the board. e.g. A1, A2, and A3 Connect the voltmeters across each of the resistors and the fourth one should be connected across the batteries Learners will record the readings on each voltmeter. Total potential difference is calculated from V1, V2, and V3 and the sum is compared to potential difference across the batteries VT = V1 + V2 + V3 Homework: 30 min 2.3 Conclusion Chalkboard / whiteboard summary concludes the lesson , stating that resistors in series are: o Potential dividers o Current is the same o Total resistance increases Report writing skills can be practiced from time to time using demonstrations available. Term 2 Page 67 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 68 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans GRADE 10 SUBJECT PHYSICAL SCIENCES LESSON SUMMARY FOR: DATE STARTED: WEEK 19 TOPIC 122222 RESISTORS IN PARALLEL 2 – TIME: 60 MINUTES Lesson 4 DATE COMPLETED: 1. Learners will be taught and learn the following concepts: Effect of resistors in series Total resistance in a circuit Potential difference across each resistor and total potential difference LESSON OBJECTIVES 2. The outcomes of the lesson are : At the end of the lesson learners should be able to : Measure resistance of each resistor and calculate the total resistance of the circuit Measure potential difference across of each resistor and calculate the total potential difference in a circuit Describe the effect of resistors connected in series TEACHER ACTIVITIES 1. LEARNER ACTIVITIES 1. Teaching methods Baseline Demonstration , Investigative & Question and answer Activity 1 2. Lesson development: 2.1 Introduction 1.1 Define resistance 1.2 How should ammeter and voltmeter be connected in a circuit 1.3 What is the relationship between current and resistance in a circuit? 1.3 Measure current on each resistor and record results 1.4 Measure potential difference on each one of the resistors and record the results a. Pre-knowledge required. Resistance, calculations and current Potential difference b. Baseline assessment Refer to learner activities c. RESOURCES NEEDED Baseline: 5 min o 3 Resistors o Batteries o 3 Ammeters o 4 Voltmeter o Switch Do corrections on the board explaining and clarifying misconceptions. 2.2 Main Body (Lesson presentation) TIMING 2.2 Demonstration From the previous lesson summary : resistors in PARALLEL are: o Current dividers o Potential difference is the same o Total resistance decreases. Effective resistance of resistors in parallel is lower Activity 2 Demonstration than the resistance in the smallest resistor and the circuit diagram, calculations to find resistance, current or potential difference can be done using 1. Find the current in the 20Ω and 5Ω resistors in the following circuit. Term 2 Page 69 30 min © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans R = V Answering questions I 10 min Equation for the calculation of the parallel resistances R1 and R2: Corrections: 10 min Provide learners with a question to demonstrate manner in which calculations should be done in physical science. 2. In the circuit below, the reading on the ammeter is 3.2 A. Conclusion: 5 min Example 1 Homework: 30 min Determine: a. the reading on the voltmeter; a) b) c) d) Calculate the effective resistance of the parallel combination Determine the potential difference on V2 How much is the potential difference through R1? Find potential difference across terminals of the battery Insist that learners should identify given quantities, what is required to be calculated and the relevant equation Transcribe the equation to the board and learners do the same on their books b. c. the potential difference across the 40Ω resistor; and the current in the 40Ω resistor. Term 2 Page 70 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Substitute without changing subject of the formula 3. Indicate mark allocation and emphasise the importance of the unit Example 2 Use the diagram below and calculate: a) total resistance in a circuit b) current in A1 c) How does current in A1 relate to current in A2 ? d) Determine V1 e) Where do you think potential difference will be lesser? V1 or V2? Give a reason For the circuit above: a. Determine the total resistance. b. Find the reading on the ammeter. c. Draw a voltmeter in the correct place to measure the potential difference across the 0.3Ω resistor. d. Draw an ammeter in the correct place to measure the current in the 0.3Ω resistor. e. Determine the readings on the meters mentioned in parts (c) and (d) above. 2.3 Conclusion Chalkboard / whiteboard summary concludes the lesson, showing how calculations can be done in physical sciences. Learners are reminded of importance of transcribing and substituting without changing subject of the formula 4) Explain, step by step, how to calculate the amount of current (I) that will go through each resistor in this parallel circuit, and also the voltage (V) dropped by each resistor: Term 2 Page 71 © Gauteng Department of Education (ver.1) Grade 10 Physical Sciences Lesson Plans Reflection/Notes: Name of Teacher: HOD: Sign: Sign: Date: Date: Term 2 Page 72 © Gauteng Department of Education (ver.1)