4.4 - Dilution
advertisement

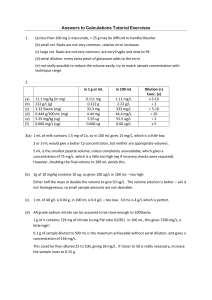
4.4 - Dilution December 6, 2017 9:59 AM Dilution is the process of preparing a less concentrated solution from a more concentrated one. The formula for dilution is 1 = concentrated solution (or stock solution) 2 = diluted solution Example 1: If 32 mL stock solution of 6.5 M H2SO4 is diluted to a volume of 500mL. What would be the resulting concentration? 4 - Solutions Page 1 500mL. What would be the resulting concentration? Example 2: If 45.3mL of stock solution is diluted to a 200mL 0.0300M solution. What would be the concentration of the stock solution? Example 3: Ms. Ng has a 1.000L stock solution of copper (II) sulphate pentahydrate. 100mL of solution is obtained and diluted to a 1.000L 0.500M solution. How many grams of copper (II) sulphate pentahydrate crystals is originally used to prepare the stock solution? 4 - Solutions Page 2