Organic Chemistry Study Guide: Alkynes & Substitution
advertisement

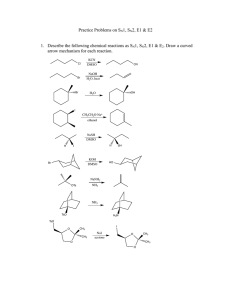
Exam 4 Study Guide Alkynes (Chp 7) and Nucleophilic Substitution and -Elimination (Chp 9) The following topics will be covered on the exam. Know them. Chapter 7 Nomenclature of alkynes; draw structure of an alkyne when given IUPAC name, or vice versa. Know the following reactions (know the reaction steps, not electron movement: Preparation of alkynes: Alkylation with alkyne anions with methyl and primary haloalkanes From alkenes—dehydrohalogenation Hydration of alkynes to aldehydes and ketones Hydroboration/oxidation Acid-catalyzed hydration Reduction of Alkynes Catalytic reduction (metals, Lindlar’s catalyst) Hydroboration-protonolysis Dissolving-metal reduction Know the following reactions and stereochemistry: Electrophilic Addition to Alkynes Addition of Br2 and Cl2 Addition of HBr and HCl Synthesis—be able to provide a multi-step synthesis of a target molecule Chapter 9 Nucleophilic substitution in haloalkanes SN2 mechanism o bimolecular reaction o transition state o energy diagram o stereochemistry SN1 mechanism o unimolecular reaction o transition state/carbocation formation o energy diagram o stereochemistry Criteria for deciding between an SN1 and an SN2 occurring o Electronic vs steric o Carbocation stability and rearrangement (SN1) o Leaving group stability o Solvent effects o Strength of the nucleophile o Kinetics o Summarize SN1 vs SN2 rxn for haloalkanes 1 -Elimination o Zaitsev products o E1 vs E2 mechanism Uni- vs bimolecular Intermediate vs transition state Energy diagrams Kinetics Stereochemistry o Summarize E1 vs E2 rxn for haloalkanes Be able to determine the results of the competition between substitution and elimination reactions, writing all resulting products (indicating major and minor) Practice Problems: 1. Draw structural formulas for the major product(s) formed in the reaction of 3-hexyne with each of the following reagents a. xs H2/Pt b. Na in NH3(l) c. NaNH2 in NH3(l) d. BH3 followed by CH3COOH e. HBr (1 equivalent) f. H2O in H2SO4/HgSO4 2. Draw the structure for the enol and ketone/aldehyde resulting from the following reactions. HgSO a. CH3(CH2)5C CH + H2O H SO 4 2 4 (sia)2BH b. CH3(CH2)5C CH + H2O NaOH/H2O2 3. What reagents and experimental conditions would you use to convert propyne into the products below? (more than one step may be required) a. b. c. 2 d. 4. Show reagents to bring about each conversion: 5. From each pair select the strongest nucleophile a. H2O or OHb. Cl- or I- in methanol c. Cl- or I- in DMSO d. CH3OCH3 or CH3SCH3 6. Draw the structural formula for the product of each SN2 reaction. a. CH3CH2CH2Cl + CH3CH2O- Na+ ethanol b. (CH3)3N + CH3I c. acetone + NH3 ethanol 7. Draw the structural formula for the product of each SN1 reaction. a. + CH3CH2OH ethanol S enantiomer b. + CH3OH methanol 8. Draw structural formulas for the alkene(s) formed by treatment with sodium ethoxide in ethanol. Assume an E2 mechanism. a. b. 3 c. 9. To which mechanism (SN1, SN2, E1, E2) does each statement apply? a. Involves a carbocation intermediate. b. Is first order in haloalkane and first order in nucleophile. c. Involves inversion of configuration at the site of substitution. d. Involves retention of configuration at the site of substitution. e. Substitution at a stereocenter gives predominantly a racemic product. f. Is greatly accelerated in protic solvents with increasing polarity. g. Order of reactivity of haloalkanes is 3º>2º >1º. h. Order of reactivity of haloalkanes is methyl>1º>2º >3º. 10. Draw structural formulas for the organic product(s) of the following reactions and specify the likely mechanism. a. b. + CH3OH + methanol DMSO (R)-2-chlorobutane c. + CH3O-Na+ methanol 11. Show how to convert the starting material into the desired product (might be more than one step). a. b. c. d. e. f. 4