Biology Study Guide: Macromolecules, Bonds, Enzymes
advertisement

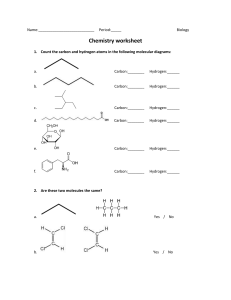
Unit 3 Study Guide Name: ___________________________ Period: ______ 1. Complete the chart below. List all examples from your notes. Macromolecule Monomers Polymers Carbohydrates Proteins Lipids Nucleic acids 2. What is the difference between an ionic bond and a covalent bond? ____________________________________ __________________________________________________________________________________________________ 3. What type of bond is likely to form between metals and nonmetals? ________________________________ 4. Draw and label a phospholipid. Identify which end is hydrophilic and which is hydrophobic. 5. Complete the following table. Identify the biological molecule structure illustrated below. Structure Type of Biological Molecule Is this a monomer or polymer? 6. Label the diagram to show the solution, solute, and solvent. Which substance does the dissolving in a solution? 7. Complete the chart below. Element # Particles (Proton, Neutrons, Electrons) Protons = 6 Neutrons = 6 Carbon Electrons = 6 Atomic Model Diagram Protons = Hydrogen Neutrons = Electrons = Protons = Oxygen Neutrons = Electrons = 8. Complete the chart below using information from the ‘Testing an Unknown Substance’ Lab. Biological Molecule Disaccharides Polysaccharides Protein Lipids Test Positive Color Indicator Biuret’s Test Translucent 9. What substance did we use as a ‘negative control’ in the Biomolecule Lab (‘Testing an Unknown Substance’) __________________________ 10. Why do molecules form hydrogen bonds? ____________________________________________________________________________________ 11. What are the three properties of water that are related to hydrogen bonds? a. __________________________ b. __________________________ c. __________________________ 12. Which property of water is responsible for surface tension? ___________________________ 13. Which property of water is responsible for allowing plants to transport water from their roots to their leaves? _____________________________________ 14. Which property of water helps living things regulate their temperature? ______________________ 15. Why do fats and oils rarely dissolve in water? __________________________________________ 16. Label the pH scale below as either acidic, basic, or neutral. 17. What is the lock-and-key model? ____________________________________________________________________________________ ____________________________________________________________________________________ 18. What is the difference between dehydration synthesis and hydrolysis? ____________________________________________________________________________________ ____________________________________________________________________________________ 19. What is denaturation? ____________________________________________________________________________________________ ____________________________________________________________________________ 20. Complete the following chart by drawing a graph to illustrate the effect of each condition on enzyme activity. Condition Temperature pH Substrate concentration Graph